
IPRATROPIUM BROMIDE/SALBUTAMOL CIPLA 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION


How to use IPRATROPIUM BROMIDE/SALBUTAMOL CIPLA 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Ipratropium Bromide/Salbutamol Cipla and what is it used for
- What you need to know before you use Ipratropium Bromide/Salbutamol Cipla
- How to use Ipratropium Bromide/Salbutamol Cipla
- Possible Adverse Effects
- Storage of Ipratropium Bromide/Salbutamol Cipla
- Package Contents and Additional Information
Introduction
Package Leaflet: Information for the User
Ipratropium Bromide/Salbutamol Cipla 0.5mg/2.5mg Solution for Inhalation by Nebuliser
Ipratropium bromide/salbutamol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Ipratropium Bromide/Salbutamol Cipla and what is it used for
- What you need to know before you use Ipratropium Bromide/Salbutamol Cipla
- How to use Ipratropium Bromide/Salbutamol Cipla
- Possible side effects
- Storage of Ipratropium Bromide/Salbutamol Cipla
- Contents of the pack and further information
1. What is Ipratropium Bromide/Salbutamol Cipla and what is it used for
This medicine is called Ipratropium Bromide/Salbutamol Cipla. The active substances are ipratropium bromide and salbutamol. Ipratropium bromide and salbutamol belong to a group of medicines called bronchodilators, which help to improve breathing by opening up the airways. They do this by preventing the contraction of the smooth muscle that surrounds the airways, so that they stay open. Ipratropium bromide works by blocking the nerve signals that are sent to the muscles that surround the airways, and salbutamol works by stimulating the β2 receptors in the muscles.
Ipratropium Bromide/Salbutamol Cipla is used to treat breathing problems in patients over 12 years old with long-term breathing difficulties (chronic obstructive pulmonary disease such as chronic bronchitis, emphysema). Ipratropium Bromide/Salbutamol Cipla will relieve wheezing, shortness of breath and chest tightness.
This medicine is used with a device called a "nebuliser", which turns the medicine into a mist that you can breathe in.
2. What you need to know before you use Ipratropium Bromide/Salbutamol Cipla
Do not use Ipratropium Bromide/Salbutamol Cipla:
- if you are allergic to salbutamol, ipratropium bromide, atropine (including atropine-like medicines) or any of the other ingredients of this medicine (listed in section 6),
- if you have cardiomegaly (enlarged heart) or a disease called hypertrophic obstructive cardiomyopathy or HOCM,
- if you have a fast heart rate (tachycardias),
Warnings and precautions
Tell your doctor before you start using ipratropium/salbutamol:
- if you have or think you may have a condition called glaucoma (increased pressure in the eyes) or any other eye condition. Your doctor may advise you to protect your eyes when using this medicine.
- if you are a man and have an enlarged prostate or problems passing urine.
- if you have recently had a heart attack (myocardial infarction).
- if you have arterial problems or leg pain when walking.
- if you have a history of heart disease, irregular heart rhythm or angina pectoris (please inform your doctor before starting to use this medicine).
- if you have diabetes.
- if you have an overactive thyroid gland.
- if you have cystic fibrosis.
- if you have been told you have a tumour on your adrenal gland.
- if you have a condition called phaeochromocytoma, which is a rare, non-cancerous tumour. Using the inhaler may make the symptoms of this condition worse.
- if the liquid or mist accidentally gets into your eyes, you may feel pain, stinging or redness, your pupils may become larger, your vision may become blurred, you may see colours or lights. If this happens, tell your doctor. If you have eye problems at any other time, tell your doctor.
There have been reports of tooth decay with the use of salbutamol. It is recommended, especially in children, to pay attention to good oral hygiene and have regular dental check-ups.
Lactic acidosis associated with high therapeutic doses of salbutamol has been observed, mainly in patients treated for acute bronchospasm (see sections 3 and 4). The increase in lactate levels can lead to lack of breathing and hyperventilation. Talk to your doctor immediately if you feel that the medicine is not working as usual and you need to use the nebuliser more often than your doctor has recommended.
Tell your doctor if your breathing problems get worse or if the medicine does not relieve your breathing problems as well as before or if you are using the short-acting "rescue" inhaler more often than usual.
If you use high doses of ipratropium/salbutamol for a long time, you should have your blood potassium levels checked, especially if you are taking other medicines at the same time, such as steroids (corticosteroids), medicines that increase urine production (diuretics) or other medicines that open up the airways, such as theophylline (xanthines).
Children and adolescents
This medicine must not be used in children under 12 years old.
Other medicines and Ipratropium Bromide/Salbutamol Cipla
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Certain medicines may interact with ipratropium bromide/salbutamol and make the side effects worse or reduce the effect of ipratropium/salbutamol. Always tell your doctor if you are taking any of the following medicines:
- Other medicines that help you breathe, such as salbutamol and preventers such as beclometasone dipropionate. These may increase the effect of ipratropium bromide/salbutamol and increase the intensity of the side effects.
- Betablockers, i.e. medicines used to treat heart conditions, such as chest pain that occurs during exercise (called angina pectoris), irregular heartbeats (or arrhythmias) and high blood pressure (called hypertension). Some of them, such as propranolol, may reduce the amount of potassium in your blood when given at the same time as ipratropium bromide/salbutamol (betablockers may reduce the effect of salbutamol).
- Certain medicines for treating depression ("antidepressants"). These include monoamine oxidase inhibitors (e.g. phenelzine) or tricyclic antidepressants (e.g. amitriptyline).
- Digoxin (for heart problems) may cause heart rhythm problems when given with ipratropium bromide/salbutamol.
- Medicines called "anticholinergics". These are used to treat colic, Parkinson's disease, problems passing urine or faecal or urinary incontinence.
- A reduction in the amount of potassium in the blood (hypokalaemia) due to ipratropium/salbutamol is more likely if you are using ipratropium/salbutamol with other treatments for asthma, with inhaled or oral corticosteroids (such as prednisone), or with diuretics (to increase urine production). Low blood potassium levels can cause muscle weakness, muscle contractions or heart rhythm abnormalities. Your doctor may do a blood test to measure your potassium levels from time to time.
- Anaesthetics may increase the tendency to experience the side effects of salbutamol on the heart. Your doctor will closely monitor you or may decide to stop treatment with ipratropium/salbutamol if you are going to have an operation.
If you are going to have a general anaesthetic in hospital, tell the anaesthetist about the medicines you are taking.
Using Ipratropium Bromide/Salbutamol Cipla with food and drink
Food and drink do not affect this medicine.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not use ipratropium/salbutamol if you are pregnant, unless your doctor decides that the benefit to you is greater than the risk to your baby.
This medicine can be used during breast-feeding. Ask your doctor, pharmacist or nurse for advice before using this medicine during breast-feeding.
Driving and using machines
If you experience side effects such as dizziness, difficulty focusing and blurred vision during treatment with ipratropium/salbutamol, you should avoid tasks that may be potentially hazardous, such as driving or using machines.
Ipratropium Bromide/Salbutamol Cipla contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, and is therefore essentially "sodium-free".
3. How to use Ipratropium Bromide/Salbutamol Cipla
This medicine is for inhalation use. The solution for inhalation by nebuliser is for oral inhalation after nebulisation.
Follow exactly the instructions for administration of this medicine given by your doctor. If you are in doubt, ask your doctor or nurse.
This medicine should be used as needed and not on a regular basis.
If your asthma is active (for example, you have symptoms or frequent attacks, such as shortness of breath that makes it difficult to speak, eat or sleep, coughing, wheezing, chest tightness or limited physical ability), you should tell your doctor immediately, who may start you on a medicine or increase the dose of treatment, such as an inhaled corticosteroid, to control your asthma.
Tell your doctor as soon as possible if your medicine seems to be not working as well as usual (for example, if you need higher doses to relieve your breathing problems or if your inhaler does not provide relief for at least 3 hours), as your asthma may be getting worse and you may need a different medicine.
If you use this medicine more than twice a week to treat your asthma symptoms, not including preventive use before exercise, this indicates poorly controlled asthma and may increase the risk of severe asthma attacks (worsening of asthma) that can have serious complications and can be life-threatening. You should contact your doctor as soon as possible to review your asthma treatment.
If you use a medicine to prevent inflammation in your lungs every day, e.g. an "inhaled corticosteroid", it is important that you continue to use it regularly, even if you feel better.
The recommended dose for adults and children over 12 years oldis 1 ampoule, 3 to 4 times a day.
Elderly patients should take the usual adult dose.
Use in children
Do notuse this ipratropium/salbutamol in children under 12 years old.
Do not swallow or give this medicine by injection.
The label will tell you how much to take and how often.
Do not use more of this medicine than your doctor has told you. Tell your doctor if your breathing problems get worse, if the medicine does not relieve your breathing problems as well as before or if you are using the short-acting "rescue" inhaler more often than usual.
This medicine should be used with a suitable nebuliser, e.g. PARI LC PLUS, pneumatic nebuliser. Read the instructions for use of the nebuliser in the PARI LC PLUS leaflet carefully before starting inhalation.
Instructions for use
- Prepare the nebuliser according to the manufacturer's instructions and your doctor's advice.
- Open the bag and remove the strip of single-dose ampoules.
- Carefully separate one ampoule from the labelled strip by twisting and pulling. Never use an ampoule that has already been opened or if the solution for inhalation by nebuliser is discoloured (diagram A).
- Do not use it if it has already been opened or if the liquid inside is discoloured.
- Hold the ampoule upright and open it by twisting the top (diagram B).
- Transfer all the contents to the nebuliser chamber by squeezing the ampoule (diagram C).
- Assemble the nebuliser and use it according to the manufacturer's instructions and your doctor's advice.
- If your doctor has told you that your medicine needs to be diluted, you will be given a sterile sodium chloride solution 0.9%. Your doctor will explain what to do.
- After using the nebuliser, discard any solution that is left in the chamber. If there is any solution for inhalation by nebuliser left in the ampoule, it should also be discarded.
- Clean the nebuliser thoroughly according to the manufacturer's instructions.
A | B | C |
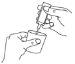

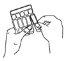
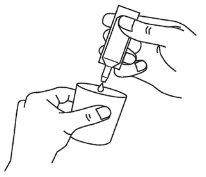
Do not dilute the solution for inhalation by nebuliser or mix it with other medicines, unless your doctor tells you to.
The single-dose ampoules of this medicine do not contain preservatives and, therefore, it is important that the contents are used immediately after opening. You should use a new ampoule for each administration of ipratropium/salbutamol with the nebuliser.
Partially used, opened or damaged ampoules should be discarded. You should neveruse an ampoule that has already been opened.
It is important that you follow these instructions to avoid contaminating the solution for inhalation by nebuliser contained in the ampoules.
Do notswallow the solution for inhalation by nebuliser or use it for injections.
Do notlet the solution for inhalation by nebuliser or the mist get into your eyes. If any of the liquids or mist accidentally get into your eyes, you may feel pain, stinging or redness, your pupils may become larger, your vision may become blurred, you may see colours or lights. If this happens, tell your doctor. If you have eye problems at any other time, tell your doctor.
If you use more Ipratropium Bromide/Salbutamol Cipla than you should
If you have taken a slightly higher dose than usual, you may notice a faster heart rate (palpitations) or tremors. Other symptoms may include chest pain, changes in blood pressure, shortness of breath, restlessness or dizziness. These effects usually go away after a few hours. You may have a lower level of potassium in your blood; your doctor may want to check your potassium level by doing a blood test from time to time. Tell your doctor if you are worried about any of these symptoms or if they persist.
If you use more of this medicine than you should, tell your doctor immediately or go to the nearest hospital. If you decide to go to a doctor or hospital, you should take all your medicines, including those you bought without a prescription, with you in their original packaging if possible. Also, take this leaflet with you to show to the doctor.
In case of overdose or accidental ingestion, contact your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, stating the medicine and the amount taken.
If you forget to use Ipratropium Bromide/Salbutamol Cipla
If you forget to take a dose at the right time, take it as soon as you remember. Do not take a double dose to make up for forgotten doses.
If you stop using Ipratropium Bromide/Salbutamol Cipla
Your doctor will tell you how long to use Ipratropium Bromide/Salbutamol Cipla. Do not stop using Ipratropium Bromide/Salbutamol Cipla without talking to your doctor first.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. Some adverse effects may be serious and require medical intervention.
Severe Adverse Effects
- If your respiratory problem or wheezing worsens immediately after inhaling this medicine, or you have difficulty breathing and become short of breath, do not take more ipratropium/salbutamol; use your short-acting relief inhaler immediately. You should stop using ipratropium/salbutamol and contact your doctor as soon as possible. Your doctor may prescribe an alternative treatment for your condition.
- If you think you are allergic to ipratropium/salbutamol or are having an allergic reaction to the nebulizer solution (including swelling of the tongue, lips, and face), stop using this medicine and contact your doctor immediately.
Other Adverse Effects May Occur with the Following Frequencies:
Frequent(may affect up to 1 in 10 people)
- Dry mouth
- Nausea (feeling sick)
- Irritation of the mouth and throat
Infrequent(may affect up to 1 in 100 people)
- Headache
- Dizziness
- Nervousness
- Tremors
- Feeling of dizziness or spinning (vertigo)
- Palpitations (strong heartbeat)
- Fast heartbeat
- Cough
- Throat irritation
- Speech problems
- Difficulty urinating
- Skin reactions
Rare(may affect up to 1 in 1,000 people)
- Severe allergic reaction causing difficulty breathing or dizziness
- Allergic reaction with hives and itching
- Swelling of the face, lips, and tongue
- Low potassium levels
- Mental disorders
- Sweating
- Eye pain or other eye problems, such as blurred vision, mydriasis (excessive pupil dilation), and glaucoma (increased eye pressure)
- Irregular heartbeats
- Low blood pressure
- Heart failure
- Respiratory problems and difficulty breathing
- Throat swelling
- Diarrhea, constipation, vomiting, or other digestive problems
- Taste disturbance
- Tooth decay
- Muscle pain
- Weakness and cramps
- Throat dryness
- Mouth edema
- Stomatitis
- Feeling weak
- Mood changes
- Difficulty urinating
Very Rare(may affect up to 1 in 10,000 people)
- High blood pressure
Frequency Not Known(frequency cannot be estimated from the available data)
A condition known as lactic acidosis, which can cause stomach pain, hyperventilation, difficulty breathing, despite possible improvement in wheezing, cold hands and feet, irregular heartbeat, or thirst.
Although the exact frequency is unknown, some people may experience chest pain (due to problems such as angina). Inform your doctor as soon as possible if you experience these symptoms while receiving treatment with ipratropium/salbutamol, but do not stop using this medicine unless your doctor tells you to.
You may also have an unusually low level of potassium in your blood ("hypokalemia"). If you have hypokalemia, your doctor will continue to monitor your potassium levels.
Reporting Adverse Effects
If you experience any adverse effects, consult your doctor or pharmacist, even if they are possible adverse effects not listed in this leaflet. You can also report them directly through the Spanish Medicines Surveillance System for human use: www.notificaRAM.es. By reporting adverse effects, you can help provide more information on the safety of this medicine.
5. Storage of Ipratropium Bromide/Salbutamol Cipla
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date stated on the carton, pouch, and ampoule label after CAD. The expiration date is the last day of the month indicated.
Do not refrigerate or freeze. Do not store above 25°C.
For single use. Use immediately after opening the ampoule for the first time. Discard immediately after the first use.
Disposal of partially used, opened, or damaged ampoules will be carried out in accordance with local regulations.
Store the ampoules in the aluminum wrapper or outer packaging to protect them from light.
Do not use this medicine if you notice that the nebulizer solution is cloudy.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Ipratropium Bromide/Salbutamol Cipla
- The active ingredients are ipratropium bromide and salbutamol. Each single-dose ampoule (2.5 mL dose) contains 0.5 mg of ipratropium bromide (equivalent to 525 micrograms of ipratropium bromide monohydrate) and 2.5 mg of salbutamol (as sulfate).
- The other excipients are sodium chloride, water for injection, and sulfuric acid (for pH adjustment).
Appearance of the Product and Package Contents
The single-dose package is a polyethylene ampoule containing 2.5 mL of clear and colorless nebulizer solution.
Five plastic ampoules in an aluminum wrapper with triple lamination (polyester film/aluminum foil/polyethylene film) and packaged in cardboard boxes containing 10, 20, 40, 60, 80, or 100 ampoules.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Cipla Europe NV
De Keyserlei 58-60, Box-19,
2018 Antwerp
Belgium
Manufacturer
Cipla Europe NV, De Keyserlei 58-60, Box-19, 2018, Antwerp, Belgium
or
ALTERNO LABS d.o.o., Brnciceva ulica 29, Ljubljana-Crnuce, 1231, Slovenia
Local Representative
Cipla Europe NV, Spanish branch,
C/ Guzmán el Bueno, 133 Edif Britannia-28003- Madrid
This Medicine is Authorized in the Member States of the European Economic Area with the Following Names:
Netherlands: Zerseos 0.5 mg/2.5 mg per 2.5 ml, nebulizer solution
Germany: Ipratropium/Salbutamol Cipla 0.5 mg / 2.5 mg solution for inhalation
Spain: Ipratropio bromuro/Salbutamol Cipla 0.5 mg/2.5 mg nebulizer solution
Ireland: Zerseos 0.5 mg/2.5 mg per 2.5 ml nebuliser solution
Poland: Ipratropium/Salbutamol Cipla, (0.5 mg + 2.5 mg)/2.5 ml, nebulizer solution
Date of the Last Revision of this Leaflet:January 2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price13.88 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IPRATROPIUM BROMIDE/SALBUTAMOL CIPLA 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATIONDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Genetic S.P.A.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Neutec Inhaler Ireland LimitedPrescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for IPRATROPIUM BROMIDE/SALBUTAMOL CIPLA 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION
Discuss questions about IPRATROPIUM BROMIDE/SALBUTAMOL CIPLA 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















