
Ipratropium /salbutamol Cipla

Ask a doctor about a prescription for Ipratropium /salbutamol Cipla

How to use Ipratropium /salbutamol Cipla
Leaflet accompanying the packaging: information for the user
Ipratropium /Salbutamol Cipla, (0.5 mg + 2.5 mg)/2.5 ml, solution for nebulization
Ipratropium bromide + Salbutamol
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Ipratropium /Salbutamol Cipla and what is it used for
- 2. Important information before using Ipratropium /Salbutamol Cipla
- 3. How to use Ipratropium /Salbutamol Cipla
- 4. Possible side effects
- 5. How to store Ipratropium /Salbutamol Cipla
- 6. Contents of the packaging and other information
1. What is Ipratropium /Salbutamol Cipla and what is it used for
The name of the medicine is Ipratropium /Salbutamol Cipla. The active substances of the medicine are ipratropium bromide and salbutamol sulfate. Ipratropium bromide and salbutamol belong to a group of medicines called bronchodilators, which make breathing easier by widening the airways. This happens because they prevent the airway muscles from contracting, keeping them open. Ipratropium bromide works by blocking nerve signals to the muscles surrounding the airways, while salbutamol works by stimulating beta receptors in these muscles.
Ipratropium /Salbutamol Cipla is used to treat breathing problems in patients over 12 years old with long-term breathing difficulties (chronic obstructive pulmonary disease, such as chronic bronchitis, emphysema). Ipratropium /Salbutamol Cipla reduces wheezing, shortness of breath, and chest tightness.
The medicine is used with a device called a nebulizer. The nebulizer turns the medicine into an aerosol that the patient will inhale.
2. Important information before using Ipratropium /Salbutamol Cipla
When not to use Ipratropium /Salbutamol Cipla:
- if the patient is allergic to salbutamol, ipratropium bromide, atropine (including medicines similar to atropine) or any of the other ingredients of this medicine (listed in section 6).
- if the patient has a enlarged heart or a disease called hypertrophic cardiomyopathy with outflow obstruction.
- if the patient has a very fast heart rate (called tachyarrhythmia).
Warnings and precautions
Before starting to use Ipratropium /Salbutamol Cipla, the patient should discuss it with their doctor:
- if the patient has or is suspected to have a eye disease called glaucoma (increased pressure in the eyeball) or any other eye problems. The doctor may recommend eye protection during use of Ipratropium /Salbutamol Cipla;
- if the patient (male) has an enlarged prostate or problems urinating;
- if the patient has recently had a heart attack;
- if the patient has vascular problems or leg pain when walking;
- if the patient has heart disease, irregular heartbeat, or angina pectoris (should inform the doctor before starting to use this medicine);
- if the patient has diabetes;
- if the patient has hyperthyroidism;
- if the patient has cystic fibrosis;
- if the patient has an adrenal gland tumor;
- if the patient has ever had an adrenal gland tumor - a rare type of non-cancerous tumor. Using an inhaler can worsen its symptoms;
- if the solution or resulting aerosol accidentally gets into the eyes, it may cause painful stinging or eye redness, pupil dilation, blurred vision, or seeing a colored halo around light sources or colored spots. In such a case, the patient should consult a doctor. If eye problems occur at any other time, the patient should consult a doctor.
There have been reports of tooth decay after using salbutamol. It is recommended, especially in children, to take good care of oral hygiene and have regular dental check-ups.
There have been reports of a condition called lactic acidosis associated with high therapeutic doses of salbutamol, mainly in patients being treated for acute bronchospasm (see sections 3 and 4). Elevated lactate levels can cause shortness of breath and hyperventilation, even if wheezing has decreased. If the patient notices that the medicine is not working as well as usual and that they need to use the nebulizer more often than prescribed by the doctor, they should consult their doctor immediately.
The patient should contact their doctor immediately if they experience sudden worsening of breathing difficulties or if the prescribed dose does not have the usual effect. The patient should not increase the dose of the medicine without consulting their doctor.
If the patient uses large doses of Ipratropium /Salbutamol Cipla for a long time, they should have their blood potassium levels checked, especially if they are taking other medicines at the same time, such as steroids (corticosteroids), diuretics, or other bronchodilators, such as theophylline (xanthine).
Children and adolescents
Ipratropium /Salbutamol Cipla should not be used in children under 12 years old.
Ipratropium /Salbutamol Cipla and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Some medicines may interact with Ipratropium /Salbutamol Cipla and may increase the risk of side effects or decrease the effect of Ipratropium /Salbutamol Cipla. The patient should always inform their doctor if they are taking any of the following medicines:
- other bronchodilators, such as salbutamol, or anti-inflammatory medicines, such as beclomethasone dipropionate. They may increase the effect of Ipratropium /Salbutamol Cipla and increase the risk of side effects.
- beta-blockers, i.e. medicines usually used to treat heart conditions, such as exertional chest pain (angina pectoris), irregular heartbeat, or arrhythmias and high blood pressure (hypertension). They include medicines such as propranolol, which may decrease potassium levels in the blood if given together with Ipratropium /Salbutamol Cipla (beta-blockers may decrease the effect of salbutamol).
in the blood, especially if the patient is taking other medicines at the same time, such as steroids (corticosteroids), diuretics, or other bronchodilators, such as theophylline (xanthine).
- certain medicines used to treat depression (antidepressants). This group includes medicines such as monoamine oxidase inhibitors (e.g. phenelzine) or tricyclic antidepressants (e.g. amitriptyline).
- digoxin (used in heart conditions) given together with Ipratropium/Salbutamol Cipla may cause arrhythmias.
- medicines called anticholinergic agents. They may be used to treat abdominal colic, Parkinson's disease, urinary incontinence, or lack of bladder or bowel control.
- there may be a decrease in potassium levels in the blood (hypokalemia) due to the effect of salbutamol - a component of Ipratropium /Salbutamol Cipla. This is more likely to occur if the patient is taking ipratropium bromide and salbutamol together with other medicines used to treat asthma, inhaled or oral steroids (such as prednisolone), or diuretics. Low potassium levels in the blood can cause muscle weakness, tremors, or arrhythmias. The doctor may recommend regular blood tests to check potassium levels.
- anesthetics may increase the sensitivity of the heart to the effect of salbutamol - the patient should be under close observation. The doctor may recommend stopping the use of Ipratropium /Salbutamol Cipla if the patient is going to have surgery.
Before planned general anesthesia in a hospital, the patient should inform the anesthesiologist about the medicines they are taking.
Ipratropium /Salbutamol Cipla with food and drink
Food and drink do not affect the use of Ipratropium /Salbutamol Cipla.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Ipratropium /Salbutamol Cipla should not be used in pregnant women unless the doctor decides that the benefits to the mother outweigh the risks to the fetus.
Ipratropium /Salbutamol Cipla can be used during breastfeeding. If the patient is breastfeeding, they should consult their doctor or pharmacist before using this medicine.
Driving and using machines
The patient should avoid activities that may be potentially dangerous, such as driving or operating machinery, if they experience side effects such as dizziness, difficulty concentrating, or blurred vision while using Ipratropium /Salbutamol Cipla.
Ipratropium /Salbutamol Cipla contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which means it is essentially 'sodium-free'.
3. How to use Ipratropium /Salbutamol Cipla
Ipratropium /Salbutamol Cipla is intended for inhalation use. The solution is intended for inhalation through the mouth during nebulization.
This medicine should always be used as directed by the doctor or pharmacist. If the patient has any doubts, they should consult their doctor or nurse.
Ipratropium /Salbutamol Cipla should be used as needed, and not regularly.
If the patient's asthma is active (e.g. frequent symptoms or exacerbations, such as shortness of breath that interferes with speaking, eating, or sleeping, coughing, wheezing, chest tightness, or limited physical activity), they should immediately tell their doctor, who may start treatment or increase the dose of a medicine that helps control asthma symptoms, such as an inhaled corticosteroid.
If the patient thinks the medicine is not working as well as usual, they should tell their doctor as soon as possible (e.g. the patient needs larger doses to relieve breathing problems or asthma symptoms do not improve for at least 3 hours after using the inhaler) because asthma may be worsening and another medicine may be needed.
If the patient uses Ipratropium /Salbutamol Cipla more than twice a week to relieve asthma symptoms, excluding preventive use before physical exertion, it means that asthma is not well controlled and the risk of severe asthma attacks (exacerbations) may increase, which can cause serious complications and be life-threatening. The patient should contact their doctor as soon as possible to verify their asthma treatment plan.
If the patient uses a medicine with anti-inflammatory effects in the lungs, such as an "inhalation corticosteroid", every day, it is essential to continue using it regularly, even if they feel better.
The recommended dose for adults and children over 12 years old is the contents of one ampoule three or four times a day.
Elderly patients should take the dose recommended for adults.
Use in children and adolescents
Ipratropium /Salbutamol Cipla should not be usedin children under 12 years old.
The medicine should not be swallowed or given by injection.
Information on what dose to take and how often to use the medicine is given on the label.
The patient should never use a larger dose of the medicine than recommended by their doctor. If breathing difficulties worsenor the medicine does not provide sufficient relief from breathing difficulties, or if the patient needs to use a short-acting inhalation bronchodilator more often than usual, they should inform their doctor.
Ipratropium /Salbutamol Cipla should be administered using an appropriate nebulizer, such as the PARI LC PLUS nebulizer or a jet nebulizer. Before starting inhalation, the patient should carefully read the instructions for using the nebulizer, provided in the leaflet accompanying the PARI LC PLUS nebulizer.
Instructions for use
- Prepare the nebulizer for use according to the manufacturer's instructions and the doctor's recommendations.
- Open the sachet and remove the blister containing the single-dose ampoules.
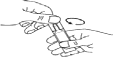
- Carefully separate the ampoule from the labeled soft blister by twisting and pulling. Never use an ampoule that has been previously opened or if the solution has changed color (Figure A).
- Do not use an ampoule that has been previously opened or contains a solution with a changed color.
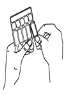
- Hold the ampoule upright and twist the top (Figure B).
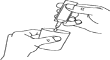
- Squeeze the contents into the nebulizer chamber (Figure C).
- Follow the manufacturer's instructions and the doctor's recommendations for assembling and using the nebulizer.
- If the doctor has informed the patient that they need to dilute the medicine, the patient will receive a sterile 0.9% sodium chloride solution. The doctor will instruct the patient on how to dilute the medicine.
- After using the nebulizer, remove any remaining solution from the chamber. The remaining solution in the ampoule should also be discarded.
- Clean the nebulizer thoroughly according to the manufacturer's instructions.
A B C
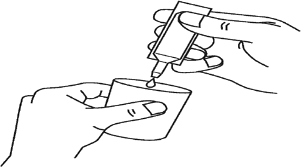
The solution should not be diluted or mixed with other medicines unless the doctor recommends it.
The single-dose ampoules of Ipratropium /Salbutamol Cipla do not contain preservatives, so it is essential to use the medicine immediately after opening the ampoule.
A new ampoule should be used each time Ipratropium /Salbutamol Cipla is used in the nebulizer.
Partially used, opened, or damaged ampoules should be discarded. Neveruse a previously opened ampoule.
It is essential to follow the above instructions to avoid contaminating the nebulization solution in the ampoule.
Do notswallow the solution or use it for injection.
Be carefulnot to get the solution or resulting aerosol into the eyes. If the solution or resulting aerosol accidentally gets into the eyes, it may cause painful stinging or eye redness, pupil dilation, blurred vision, or seeing a colored halo around light sources or colored spots. In such a case, the patient should consult a doctor. If eye problems occur at any other time, the patient should consult a doctor.
Using a higher dose of Ipratropium /Salbutamol Cipla than recommended
If a slightly higher dose than recommended is used, it may cause rapid heart rate (palpitations) or muscle tremors. Other symptoms may occur, including: chest pain, changes in blood pressure, flushing, anxiety, or dizziness. These symptoms usually resolve within a few hours. There may be a decrease in potassium levels in the blood, so the doctor may recommend regular blood tests to check potassium levels. The patient should inform their doctor if they are concerned about any of these symptoms or if they persist.
If a higher dose of the medicine is used than recommended, the patient should immediately inform their doctor or go to the nearest hospital. If a visit to the doctor or hospital is necessary, the patient should take all the medicines they are using, including those that are available without a prescription, in their original packaging. The patient should also take this leaflet to show it to the doctor.
Missing a dose of Ipratropium /Salbutamol Cipla
If a dose is missed at the scheduled time, it should be used as soon as possible. The patient should not use a double dose to make up for the missed dose.
Stopping the use of Ipratropium /Salbutamol Cipla
The doctor will tell the patient how long to use Ipratropium /Salbutamol Cipla. The patient should not stop using Ipratropium /Salbutamol Cipla without consulting their doctor first.
If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Ipratropium /Salbutamol Cipla can cause side effects, although not everybody gets them.
Some side effects may be serious and may require medical attention.
Serious side effects
- If, immediately after inhaling Ipratropium /Salbutamol Cipla, asthma symptoms or wheezing worsen or breathing becomes difficult and the patient experiences shortness of breath, they should stop using Ipratropium /Salbutamol Cipla and immediately use a short-acting inhalation bronchodilator. The patient should stop using Ipratropium /Salbutamol Cipla and immediately consult their doctor. The doctor may recommend a different treatment for the condition.
If the patient thinks they are allergic to Ipratropium /Salbutamol Cipla or suspects that they have had an allergic reaction to the solution, including swelling of the tongue, lips, and face, they should immediately stop using Ipratropium /Salbutamol Cipla and consult their doctor.
Other side effects, occurring with the following frequency:
Common, may occur in less than 1 in 10 people
- dry mouth
- nausea (vomiting)
- irritation of the mouth and throat mucosa
Uncommon, may occur in less than 1 in 100 people
- headache
- dizziness
- feeling nervous
- tremors
- feeling of dizziness or spinning (vertigo)
- palpitations (feeling of heart beating)
- rapid heart rate
- coughing
- throat irritation
- speech disorders
- urination problems
- skin reactions
Rare, may occur in less than 1 in 1000 people
- severe allergic reactions that can cause breathing difficulties or dizziness
- allergic reactions such as hives or itching
- swelling of the face, lips, and tongue
- decrease in potassium levels in the blood
- psychiatric disorders
- sweating
- eye pain or other eye problems, including blurred vision, pupil dilation (excessive pupil dilation), and glaucoma (increased pressure in the eyeball)
- heart rhythm disorders
- decrease in blood pressure
- heart failure
- breathing difficulties and shortness of breath
- throat swelling
- diarrhea, constipation, vomiting, or other gastrointestinal problems
- change in taste
- tooth decay
- muscle pain
- muscle weakness and cramps
- dry throat
- mouth swelling
- mouth mucosa inflammation
- feeling of weakness
- mood changes
- urination problems
Very rare, may occur in less than 1 in 10,000 people
- increase in blood pressure
Frequency not known(frequency cannot be estimated from the available data)
A condition called lactic acidosis, which can cause stomach pain, hyperventilation, and shortness of breath, even if wheezing has decreased, cold hands and feet, irregular heartbeat, or thirst.
In some patients, chest pain (due to conditions such as angina pectoris) may occur, although the exact frequency is unknown. If such symptoms occur while using Ipratropium /Salbutamol Cipla, the patient should immediately inform their doctor, but should not stop using the medicine unless the doctor recommends it.
There may be a significant decrease in potassium levels in the blood (hypokalemia). In such a case, the doctor may recommend regular blood tests to check potassium levels.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the patient should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Ipratropium /Salbutamol Cipla
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton, sachet, and ampoule label after: Expiry Date (EXP). The expiry date refers to the last day of the month.
Do not store above 25°C. Do not store in the refrigerator or freeze.
For single use only. Use immediately after opening the ampoule. Discard immediately after first use.
Partially used, opened, or damaged ampoules should be disposed of in accordance with local regulations.
Ampoules should be stored in the outer sachet or carton to protect them from light.
Do not use this medicine if the solution is cloudy.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Ipratropium /Salbutamol Cipla contains
- The active substances of the medicine are ipratropium bromide and salbutamol. Each 2.5 ml ampoule providing a single dose contains 0.5 mg of ipratropium bromide (as 525 micrograms of ipratropium bromide monohydrate) and 2.5 mg of salbutamol (as salbutamol sulfate).
- The other ingredients are: sodium chloride, water for injection, and sulfuric acid 1N (for pH adjustment).
What Ipratropium /Salbutamol Cipla looks like and contents of the pack
Ampoules made of LDPE, packaged in 5s in laminated sachets of Polyester/Aluminum/Polyethylene, in a carton. The packaging contains 10, 20, 40, 60, 80, or 100 ampoules of 2.5 ml of clear, colorless solution for nebulization.
Marketing authorization holder
Cipla Europe NV,
De Keyserlei 58-60,
Box-19, 2018 Antwerp,
Belgium
Importer
Cipla Europe NV, De Keyserlei 58-60, Box-19, 2018 Antwerp, Belgium
ALTERNO LABS d.o.o., Brnčičeva ulica 29, Ljubljana-Črnuče, 1231, Slovenia
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Spain:
Ipratropio bromuro/Salbutamol Cipla 0.5 mg/2.5 mg solución para inhalación por nebulizador
Netherlands:
Zerseos 0.5 mg/2.5 mg per 2.5 ml, verneveloplossing
Ireland:
Zerseos 0.5 mg/2.5 mg per 2.5 ml nebuliser solution
Germany:
Ipratropium /Salbutamol Cipla 0.5 mg / 2.5 mg Lösung für einen Vernebler
Poland:
Ipratropium /Salbutamol Cipla, (0.5 mg + 2.5 mg)/2.5 ml, solution for nebulization
Date of last revision of the leaflet:01/2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterAlterno Labs d.o.o. Cipla Europe NV
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ipratropium /salbutamol CiplaDosage form: Solution, (0.5 mg + 2.5 mg)/2.5 mlActive substance: salbutamol and ipratropium bromideManufacturer: Adamed Pharma S.A. Genetic S.p.APrescription requiredDosage form: Aerosol, (50 mcg + 21 mcg)/dose inh.Active substance: fenoterol and ipratropium bromideManufacturer: Boehringer Ingelheim Pharma GmbH & Co. KGPrescription requiredDosage form: Solution, (0.5 mg + 0.25 mg)/mlActive substance: fenoterol and ipratropium bromideManufacturer: Instituto De Angeli S.r.l.Prescription required
Alternatives to Ipratropium /salbutamol Cipla in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ipratropium /salbutamol Cipla in Spain
Alternative to Ipratropium /salbutamol Cipla in Ukraine
Online doctors for Ipratropium /salbutamol Cipla
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ipratropium /salbutamol Cipla – subject to medical assessment and local rules.














