
IPRATROPIUM BROMIDE/SALBUTAMOL NEUTEC 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION


How to use IPRATROPIUM BROMIDE/SALBUTAMOL NEUTEC 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Ipratropium bromide/Salbutamol Neutec and what is it used for
- What you need to know before starting to use Ipratropium bromide/Salbutamol Neutec
- How to use Ipratropium bromide/Salbutamol Neutec
- Possible side effects
- Storage of Ipratropium bromide/Salbutamol Neutec
- Container contents and additional information
Introduction
Leaflet: information for the user
Ipratropium bromide/Salbutamol Neutec 0.5mg/2.5mg solution for inhalation by nebulizer
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Ipratropium bromide/Salbutamol Neutec and what is it used for
- What you need to know before starting to use Ipratropium bromide/Salbutamol Neutec
- How to use Ipratropium bromide/Salbutamol Neutec
- Possible side effects
- Storage of Ipratropium bromide/Salbutamol Neutec
- Package contents and additional information
1. What is Ipratropium bromide/Salbutamol Neutec and what is it used for
Ipratropium bromide/Salbutamol Neutec contains the active ingredients ipratropium bromide and salbutamol. Both belong to a group of medications called bronchodilators, which help improve breathing by opening the airways. This is achieved by preventing the contraction of the smooth muscles surrounding the airways, allowing them to remain open.
Ipratropium bromide/Salbutamol Neutec is used in adults and adolescents from 12 years of age to treat long-term respiratory problems in a disease called "chronic obstructive pulmonary disease" or COPD.
2. What you need to know before starting to use Ipratropium bromide/Salbutamol Neutec
Do not useIpratropium bromide/Salbutamol Neutec if:
- You are allergic to salbutamol, ipratropium bromide, atropine (including atropine-like medications), or any of the other components of this medication (listed in section 6).
- You have a heart problem called "hypertrophic obstructive cardiomyopathy". This is a disease in which the wall separating the two sides of the heart thickens. The thickened heart wall can make it difficult for the heart to pump blood and block blood flow.
- You have very rapid heartbeats (what is called "tachyarrhythmia").
Warnings and precautions
Consult your doctor or pharmacist before starting to use Ipratropium bromide/Salbutamol Neutec if:
- You have glaucoma (increased eye pressure) or have been told you may have it. Your doctor will advise you to protect your eyes when using this medication.
- You have heart or circulatory problems (e.g., you feel pain in your legs when walking), or you have recently had a heart attack.
- You have diabetes.
- You have an overactive thyroid gland.
- You have problems urinating.
- You are male and have prostate problems.
- You have cystic fibrosis.
- You have had an intestinal obstruction.
- You have had a non-cancerous tumor of the adrenal gland (called "pheochromocytoma"). Using this medication can worsen symptoms.
- You have impaired liver or kidney function.
Lactic acidosis associated with high therapeutic doses of salbutamol has been observed, mainly in patients treated for acute bronchospasm (see sections 3 and 4). The increase in lactate levels can lead to lack of breathing and hyperventilation. Talk to your doctor immediately if you feel that the medication is not working as usual and you need to use the nebulizer more often than your doctor has recommended.
Consult your doctor or go to the nearest hospital immediately if your breathing difficulties worsen suddenly or if the prescribed dose does not provide as much relief as before when breathing. Do not increase the dose without your doctor's advice.
Good oral hygiene and regular dental check-ups are recommended, as Ipratropium bromide/Salbutamol Neutec can cause dry mouth, which increases the risk of tooth decay.
It is very important to avoid getting the medication in your eyes, especially if you have or are at risk of glaucoma. If you accidentally get liquid or nebulization in your eyes, you may experience eye problems, such as pain, stinging, or redness of the eyes, enlarged pupils, blurred vision, seeing colors or lights. If this happens, talk to your doctor immediately for advice. If you have eye problems at any other time, talk to your doctor for advice.
Occasionally, the use of Ipratropium bromide/Salbutamol Neutec can reduce potassium levels in the blood, especially if you are taking certain other medications at the same time. If you are at risk of this happening, your doctor may monitor the amount of potassium in your blood.
If you need to provide a urine sample as part of a routine drug test, Ipratropium bromide/Salbutamol Neutec may give a positive result in the test.
Children
This medication is not recommended for children under 12 years of age, as it has not been investigated in this age group.
Other medications and Ipratropium bromide/Salbutamol Neutec
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
In particular, tell your doctor or pharmacist if you are taking any of the following medications:
- Steroid medications such as prednisolone for the treatment of inflammatory diseases, which can increase the effect of this medication and the intensity of adverse reactions.
- Diuretics.
- Certain medications for treating anxiety and depression ("antidepressants"). This class of medications includes monoamine oxidase inhibitors (e.g., phenelzine) or tricyclic antidepressants (e.g., amitriptyline), which can increase the effect of salbutamol.
- Other medications to help you breathe, such as inhalers and tablets for asthma, and medications for the treatment of acute asthma attacks. These medications include beta-agonists (e.g., fenoterol), xanthine derivatives (e.g., theophylline or aminophylline), and anticholinergics (e.g., tiotropium), which can increase the effect of this medication and the intensity of adverse effects.
- Medications called "anticholinergics". They may be used to treat colic pain, Parkinson's disease, urinary problems, or loss of bladder or bowel control.
- Medications called "beta-blockers" such as propranolol. They may be used to treat heart problems, high blood pressure, anxiety, or migraines. Taking beta-blockers can inhibit the effects of Ipratropium bromide/Salbutamol Neutec.
- Digoxin: it is used for rapid heartbeats or heart failure, which can cause heart rhythm disorders if used with this medication.
Surgery
Some gases used in surgery (anesthetic gases) can increase the sensitivity to the adverse effects of salbutamol on the heart. If you are going to have surgery, you will be closely monitored or your doctor may decide to stop the use of Ipratropium bromide/Salbutamol Neutec. If you are about to undergo surgery, make sure to mention to your doctor, dentist, or anesthesiologist that you are taking Ipratropium bromide/Salbutamol Neutec.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medication.
Do not use Ipratropium bromide/Salbutamol Neutec if you are pregnant, unless your doctor decides that the benefit to you outweighs any risk to your child.
If you are breastfeeding, your doctor will decide whether you should stop breastfeeding or stop using this medication, taking into account the benefit of breastfeeding for the child and the benefit of treatment for you.
Driving and using machines
You may feel dizzy, have difficulty focusing, or blurred vision while taking Ipratropium bromide/Salbutamol Neutec. If this happens, do not drive or use tools or machines.
3. How to use Ipratropium bromide/Salbutamol Neutec
Follow the administration instructions for this medication exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
The recommended dose for adults and adolescents over 12 years of ageis:
the contents of 1 single-dose container, three or four times a day.
Ipratropium bromide/Salbutamol Neutec should be inhaled using a suitable nebulizer, e.g., a jet nebulizer. Read all the instructions for use of the nebulizer provided with the nebulizer before starting inhalation.
Instructions for use
Read points 1 to 5 before starting to use your nebulizer.
- Prepare your nebulizer for use according to the manufacturer's instructions and your doctor's advice.
- Open the bag and carefully separate a new container from the strip. Never use an ampoule that is already open or if the solution for inhalation by nebulizer is discolored.
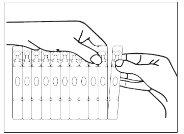
- Hold the ampoule in a vertical position and open it by twisting the top.
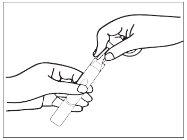
- Pressing, pour the entire contents into the nebulizer chamber, unless your doctor has given you other instructions. Make sure to use the entire contents immediately. Any unused medication should be discarded and not used.
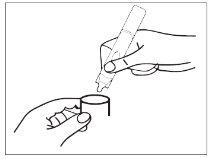
- Start and use the nebulizer according to the instructions for use. Inhale with calm and uniform breaths through the nebulizer mouthpiece/mask.
If you use more Ipratropium bromide/Salbutamol Neutec than you should
If you use more of this medication than you should, you may experience symptoms such as: dry mouth, blurred vision, rapid heartbeat, chest pain, high blood pressure, irregular heartbeats, tremors, low blood pressure, and redness of the skin. If you use more of this medication than you should, talk to a doctor or go to the nearest hospital immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to useIpratropium bromide/Salbutamol Neutec
- If you forget a dose, take it as soon as you remember.
- Do not take a double dose to make up for forgotten doses.
If you stop treatment with Ipratropium bromide/Salbutamol Neutec
Do not stop using Ipratropium bromide/Salbutamol Neutec without talking to your doctor first.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Stop usingIpratropium bromide/Salbutamol Neutecand contact your doctor immediately or go to the nearest emergency room if you notice any of the following serious side effects - you may need urgent medical treatment:
- If immediately after inhaling this medication, your respiratory problem worsens or you experience wheezing and difficulty breathing. This can occur rarely when using this medication (it can affect up to 1 in 1,000 people).
- If you have an allergic reaction: the signs can include skin rash, itching, and hives. In severe cases, the signs include swelling of the tongue, lips, and face, sudden difficulty breathing (angioedema), and a drop in blood pressure that can cause dizziness. This can occur rarely when using this medication (it can affect up to 1 in 1,000 people).
- If after taking this medication, you experience eye pain, blurred vision, or redness of the eyes, or if you see halos or colored spots. This can occur rarely when using this medication (it can affect up to 1 in 1,000 people).
Tell your doctor as soon as possible, but do not stop using this medication unless advised to do so or if you notice the following:
- Chest pain (due to heart problems, such as angina). This can occur rarely when using this medication (it can affect up to 1 in 1,000 people).
Other side effects:
Uncommon(can affect up to 1 in 100 people)
- Feeling nervous, trembling, or dizzy.
- Dry mouth.
- Cough.
- Headache.
- Feeling dizzy (nausea).
- Throat irritation.
- Increased blood pressure.
- Increased heart rate or irregular heartbeats (palpitations).
- Voice problems ("dysphonia").
- Skin reactions.
Rare(can affect up to 1 in 1,000 people)
- Irregular heartbeats.
- Decreased potassium levels in the blood (called "hypokalemia"), observed in blood tests.
- Blurred vision, dilated pupils, pain, stinging, or redness of the eyes, swelling of the eyes, seeing colors or lights.
- Increased sweating.
- Dry throat, swelling of the throat.
- Difficulty breathing or speaking due to a brief spasm of the vocal muscles.
- Diarrhea, constipation, nausea (vomiting), or other digestive problems.
- Skin rash, pruritic rash, and/or itching.
- Swelling or inflammation of the mouth.
- Muscle cramps, muscle weakness, and pain.
- Difficulty urinating.
- Feeling of weakness.
- Low blood pressure.
- Mood changes.
The following side effect may also occur, but its frequency is unknown (the frequency cannot be estimated from the available data):
- A condition known as lactic acidosis that can cause stomach pain, hyperventilation, difficulty breathing, despite possible improvement in wheezing, cold hands and feet, irregular heartbeat, or thirst.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Ipratropium bromide/Salbutamol Neutec
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the box, bag, and label of the single-dose container after CAD. The expiration date is the last day of the month indicated.
Store the single-dose containers in the outer bag and carton to protect them from light and moisture.
Do not use this medication if you notice discoloration of the liquid.
Use immediately after opening the single-dose container for the first time.
Each container is for single use.
Discard the unused contents immediately after the first use. Partially used, opened, or damaged single-dose containers should be discarded.
Medications should not be thrown down the drain or into the trash. Deposit the containers and medications you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of medications you no longer need. This will help protect the environment.
6. Container contents and additional information
What Ipratropium Bromide/Salbutamol Neutec contains
- The active substances are ipratropium bromide and salbutamol. Each single-dose container of 2.5 ml contains 0.5 mg of ipratropium bromide (as ipratropium bromide monohydrate) and 2.5 mg of salbutamol (as salbutamol sulfate).
- The other excipients are: sodium chloride, hydrochloric acid 1 N (for pH adjustment) and water for injectable preparations.
Appearance and container contents of the product
Each single-dose container contains 2.5 ml of a clear, colorless or almost colorless solution for inhalation by nebulizer.
Five single-dose containers are wrapped in a bag and packaged in a cardboard box.
Ipratropium Bromide/Salbutamol Neutec is available in packs of 10, 20, 40, 60, 80 or 100 single-dose containers.
Only some pack sizes may be marketed.
Marketing authorization holder
Neutec Inhaler Ireland Limited
22 Northumberland Road, Ballsbridge
Dublin 4, Co. Dublin
D04 ED73
Ireland
Manufacturer
NextPharma Oy
Niittyhaankatu 20
33720 Tampere
Finland
Kymos S.L.
Ronda De Can Fatjo 7b,
Parc Tecnologic Del Valles,
Cerdanyola Del Valles,
08290
Spain
This medicinal product has been authorized in the following European Economic Area member states and in the United Kingdom (Northern Ireland) under the following names:
Sweden | Ipratropium/Salbutamol Neutec |
Belgium | Ipratropium bromide/salbutamol Neutec 0.5 mg/2.5 mg verneveloplossing |
Denmark | Ipratropiumbromid/salbutamol Neutec |
Finland | Ipratropium bromide/salbutamol Neutec 0.5 mg / 2.5 mg per 2.5 ml sumutinliuos |
Germany | Ipratropiumbromid/Salbutamol Neutec 0,5 mg/2,5mg Lösung für einen Vernebler |
Iceland | Ipratropium bromide/salbutamol Neutec |
Ireland | Ipratropium bromide/salbutamol Neutec 0.5/2.5 mg per 2.5 ml nebuliser solution |
Netherlands | Ipratropiumbromide/salbutamol Neutec 0,5/2,5 mg per 2,5 ml, verneveloplossing |
Norway | Ipratropium bromide/salbutamol Neutec |
Spain | Ipratropium Bromide/Salbutamol Neutec 0.5 mg/2.5 mg solution for inhalation by nebulizer |
United Kingdom (Northern Ireland) | Ipratropium bromide/salbutamol Neutec 0.5 mg/2.5 mg per 2.5 ml nebuliser solution |
Date of last revision of this leaflet: 05/2023.
Other sources of information
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IPRATROPIUM BROMIDE/SALBUTAMOL NEUTEC 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATIONDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Genetic S.P.A.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Cipla EuropePrescription required
Online doctors for IPRATROPIUM BROMIDE/SALBUTAMOL NEUTEC 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION
Discuss questions about IPRATROPIUM BROMIDE/SALBUTAMOL NEUTEC 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















