
INSTANYL 200 micrograms NASAL SPRAY SOLUTION IN SINGLE-DOSE CONTAINER

How to use INSTANYL 200 micrograms NASAL SPRAY SOLUTION IN SINGLE-DOSE CONTAINER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Instanyl 50micrograms/dose nasal spray solution
Instanyl 100micrograms/dose nasal spray solution
Instanyl 200micrograms/dose nasal spray solution
fentanyl
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Instanyl and what is it used for.
- What you need to know before you use Instanyl
- How to use Instanyl
- Possible side effects
- Storing Instanyl
- Contents of the pack and other information
1. What is Instanyl and what is it used for
Instanyl contains the active substance fentanyl and belongs to a group of strong painkillers called opioids. Opioids work by blocking pain signals to the brain.
Instanyl acts quickly and is used to treat breakthrough pain in adult patients with cancer who are already taking opioid painkillers for their persistent pain. Breakthrough pain is a sudden pain that occurs even when you are taking your regular opioid painkillers.
2. What you need to know before you use Instanyl
Do not use Instanyl
- if you are allergic to fentanyl or any of the other ingredients of this medicine (listed in section 6);
- if you are not already using a prescribed opioid medicine every day for persistent pain (e.g. codeine, fentanyl, hydromorphone, morphine, oxycodone, meperidine), as the risk of your breathing becoming too slow and/or too shallow, or even stopping, may increase. If you have not been using these medicines, do not use Instanyl;
- if you are taking a medicine that contains sodium oxybate;
- if you have short-term pain that is not breakthrough pain;
- if you have severe breathing difficulties or severe obstructive lung disease.
- if you have had previous radiotherapy to the head or neck area;
- if you have had repeated episodes of nosebleeds.
Warnings and precautions
Keep this medicine out of the sight and reach of other people, especially children (see section 5 for more information on storing Instanyl).
Talk to your doctor or pharmacist before starting Instanyl, especially:
- if you have chronic obstructive lung disease, as Instanyl may affect your breathing rhythm.
- if you have heart problems, especially a slow heart rate, low blood pressure, or low blood volume.
- if you have liver or kidney problems.
- if you have brain problems, for example due to a brain tumour, head injury, or increased pressure in the skull.
- if you have ever had adrenal insufficiency or a lack of sex hormones (androgen deficiency) when using opioids.
- if you are taking sedatives, such as benzodiazepines or related medicines (see also section “Using Instanyl with other medicines”).
- if you are taking antidepressants or antipsychotics (see also section “Using Instanyl with other medicines”).
- if you are taking medicines called partial agonist/antagonists, e.g. buprenorphine, nalbuphine, and pentazocine (painkillers), as they may cause withdrawal symptoms. See section “Using Instanyl with other medicines” for more information.
- if you are using other nasal spray products, e.g. for the common cold or allergies.
Sleep-related breathing disorders
Instanyl may cause sleep-related breathing disorders, including sleep apnoea (pauses in breathing while sleeping) and sleep hypoxia (low oxygen levels in the blood). Symptoms may include pauses in breathing while sleeping, waking up during the night due to lack of air, difficulty staying asleep, or excessive sleepiness during the day. If you or someone else notices these symptoms, contact your doctor to consider reducing the dose.
It is very important that you contact your doctor or hospital immediately if you experience breathing difficulties while being treated with Instanyl.
Talk to your doctor if, while using Instanyl, you:
- feel pain or increased sensitivity to pain (hyperalgesia) that does not respond to a higher dose of the medicine as prescribed by your doctor.
- experience a combination of the following symptoms: nausea, vomiting, loss of appetite, fatigue, weakness, dizziness, and low blood pressure. Together, these symptoms can be a sign of a potentially life-threatening condition called adrenal insufficiency, where the adrenal glands do not produce enough hormones.
If you experience repeated nosebleeds or notice nasal discomfort during treatment with Instanyl, you should talk to your doctor, who will consider alternative treatments for your breakthrough pain.
Long-term use and tolerance
This medicine contains fentanyl, an opioid. Repeated use of opioid painkillers can make the medicine less effective (your body gets used to it, which is known as pharmacological tolerance). You may also become more sensitive to pain when using Instanyl. This is known as hyperalgesia. Increasing the dose of Instanyl may continue to reduce pain for a while, but it can also be harmful. If you notice that the medicine is becoming less effective, talk to your doctor. Your doctor will decide whether it is better to increase the dose or gradually reduce your use of Instanyl.
Dependence and addiction
Repeated use of Instanyl can also lead to dependence, abuse, and addiction, which can result in a potentially life-threatening overdose. The risk of these side effects may be greater with higher doses and longer use. Dependence or addiction can lead to a feeling of loss of control over the amount of medicine you use or how often you use it. You may feel the need to continue using the medicine even if it does not help relieve pain.
The risk of dependence or addiction varies from person to person. The risk of becoming dependent on or addicted to Instanyl may be greater if:
- you or a family member have abused alcohol, prescription medicines, or illegal drugs (“addiction”).
- you smoke.
- you have ever had mood problems (depression, anxiety, or personality disorder) or have been treated by a psychiatrist for other mental health problems.
If you notice any of the following symptoms while using Instanyl, it could be a sign of dependence or addiction.
- you need to use the medicine for longer than prescribed by your doctor.
- you need to use a higher dose than recommended.
- you are using the medicine for reasons other than those prescribed, e.g. “to feel calm” or “to help you sleep”.
- you have made repeated unsuccessful attempts to stop using the medicine or control its use.
- you feel unwell when you stop using the medicine (e.g. nausea, vomiting, diarrhoea, anxiety, shivering, shaking, and sweating), and you feel better once you take it again (“withdrawal symptoms”).
If you notice any of these signs, talk to your doctor to determine the best course of treatment for you, when it is appropriate to stop the medicine, and how to do so safely.
Children and adolescents
Instanyl should not be used in children and adolescents under 18 years of age.
Using Instanyl with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those bought without a prescription.
Instanyl can affect or be affected by other medicines.
Special care should be taken if you are being treated with any of the following medicines:
- Other painkillers and medicines for nerve pain, such as gabapentin and pregabalin.
- any medicine that makes you sleepy (has a sedative effect) such as sleeping pills, sedatives (e.g. benzodiazepines or related medicines), medicines for anxiety, antihistamines, or tranquilisers, muscle relaxants, and gabapentinoids (gabapentin and pregabalin). Using these medicines at the same time as Instanyl may cause drowsiness, deep sedation, and affect your ability to breathe (respiratory depression), which can lead to coma or even death. Therefore, concomitant use should only be considered when no other treatment options are available.
However, if your doctor prescribes Instanyl and sedatives, you should limit both the dose and duration of concomitant treatment.
Tell your doctor about all sedatives you are taking and follow their recommendations about the dose carefully. It may be helpful to inform your friends or family to be aware of the signs and symptoms mentioned above. Contact your doctor when you report such symptoms.
- any medicine that may change how your body breaks down Instanyl, such as:
- ritonavir, nelfinavir, amprenavir, and fosamprenavir (medicines that help control HIV infections);
- CYP3A4 inhibitors, such as ketoconazole, itraconazole, or fluconazole (used to treat fungal infections);
- troleandomycin, clarithromycin, or erythromycin (medicines for treating bacterial infections);
- aprepitant (used to treat severe nausea);
- diltiazem and verapamil (medicines for treating high blood pressure or heart disease).
- medicines called Monoamine Oxidase Inhibitors (MAOIs), used for severe depression, even if you have been treated with one of these in the last 2 weeks.
- the risk of side effects increases if you are taking certain antidepressants or antipsychotics. Instanyl may interact with these medicines, and you may experience changes in mental status (e.g. agitation, hallucinations, coma) and other effects such as body temperature above 38°C, increased heart rate, unstable blood pressure, and exaggerated reflexes, muscle stiffness, lack of coordination, and/or gastrointestinal symptoms (e.g. nausea, vomiting, diarrhoea). Your doctor will tell you if Instanyl is suitable for you.
- medicines called partial agonist/antagonists, e.g. buprenorphine, nalbuphine, and pentazocine (painkillers). You may experience withdrawal symptoms (nausea, vomiting, diarrhoea, anxiety, shivering, shaking, and sweating).
- other nasal spray medicines, especially oxymetazoline, xylometazoline, and similar medicines, used for relieving nasal congestion.
Instanyl with food, drinks, and alcohol
Do not drink alcohol while being treated with Instanyl, as it may increase the risk of serious side effects.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using any medicine.
Instanyl should not be used during pregnancy, unless you have discussed it with your doctor.
Instanyl should not be used during labour, as fentanyl can cause serious breathing problems in the newborn.
Fentanyl can pass into breast milk and cause side effects in the baby. Do not use Instanyl if you are breastfeeding. You should not start breastfeeding until 5 days after the last dose of Instanyl.
Driving and using machines
You should not drive or use machines while being treated with Instanyl. Instanyl can cause dizziness, drowsiness, and visual disturbances, which can affect your ability to drive or operate machinery.
3. How to use Instanyl
Before starting treatment and regularly during treatment, your doctor will also explain what you can expect from using Instanyl, when and for how long you should use it, when you should contact your doctor, and when you should stop using it (see also section 2).
Follow the instructions for administration of this medicine exactly as your doctor has told you. If you are unsure, ask your doctor or pharmacist.
The dose of Instanyl is separate from your background treatment for cancer pain.
When you start using Instanyl, your doctor will determine with you the dose that relieves your breakthrough pain.
The initial dose is one spray of 50 micrograms into one nostril each time you have a breakthrough pain episode. During the dose-finding phase, your doctor may ask you to switch to a higher dose.
If the breakthrough pain is not relieved after 10 minutes, you can use only one more spray for this episode.
In general, you should wait 4 hours before treating another episode of breakthrough pain. In exceptional cases, when a new episode occurs before 4 hours, you can use Instanyl to treat this episode, but you should wait at least 2 hours before doing so. If you regularly experience episodes of breakthrough pain separated by less than 4 hours, contact your doctor, as your regular cancer pain treatment may need to be changed.
You can use Instanyl to treat a maximum of four episodes of breakthrough pain per day.
Contact your doctor if you experience more than four episodes of breakthrough pain per day, as your regular cancer pain treatment may need to be changed.
Do not change the dose of Instanyl or your other painkillers on your own. Changes in dose should be made with your doctor.
Instanyl is for nasal use.
You should read the instructions for use at the end of this leaflet to learn how to use Instanyl.
If you use more Instanyl than you should or if you think someone has used Instanyl by accident
If you have used more Instanyl than you should, contact your doctor, hospital, or emergency service to assess the risk and advise on what to do.
Overdose symptoms are:
Drowsiness, lethargy, dizziness, decreased body temperature, decreased heart rate, difficulties with coordination of arms and legs.
In severe cases, symptoms of an overdose of Instanyl can include coma, sedation, convulsions, or severe breathing difficulties (very slow or shallow breathing). An overdose can also cause a brain disorder known as toxic leucoencephalopathy.
If you notice any of these symptoms, you should seek medical help immediately.
Note for persons taking care of the patient
If you notice that the person being treated with Instanyl acts slowly, has breathing problems, or has difficulty staying awake:
- you should seek urgent help immediately.
- while waiting for help, try to keep the person awake by talking to them or gently shaking them from time to time.
- if the person has breathing problems, try to get them to breathe every 5-10 seconds.
- if the person stops breathing, try to resuscitate them until emergency help arrives.
If you think someone has used Instanyl by accident, seek medical help immediately. Try to keep the person awake until the emergency service arrives.
If someone has used Instanyl by accident, they may experience the same symptoms as described above in case of overdose.
If you forget to use Instanyl
If you continue to have breakthrough pain, you can use Instanyl as prescribed by your doctor. If the breakthrough pain has stopped, do not use Instanyl until the next episode of breakthrough pain occurs.
If you stop using Instanyl
You should stop using Instanyl when you no longer have breakthrough pain. However, you should continue using your background painkiller for cancer pain. If you are unsure, talk to your doctor to confirm the correct dose of your regular painkiller.
You may experience sudden withdrawal symptoms similar to possible side effects of Instanyl if you stop using it. If you experience withdrawal symptoms, talk to your doctor. Your doctor will assess whether you need medicine to reduce or eliminate these symptoms.
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine may cause adverse effects, although not all people suffer from them.
Frequently, adverse effects will disappear or decrease with continued use of the medicine.
Interrupt treatment and contact your doctor, hospital, or emergency service immediately if:
- you experience a severe and sudden allergic reaction with difficulty breathing, inflammation, feeling of dizziness, increased heart rate, sweating, or loss of consciousness.
- you experience severe breathing difficulties.
- you have wheezing sounds when inhaling.
- you have convulsive pain.
- you experience extreme dizziness.
These adverse effects can be very serious.
Other adverse effects observed after using Instanyl:
Frequent(may affect up to 1 in 10 people):
Drowsiness, dizziness even with difficulty maintaining balance, headache, throat irritation, nausea, vomiting, flushing, feeling of intense heat, excessive sweating.
Uncommon(may affect up to 1 in 100 people):
Insomnia, lethargy, muscle convulsions, strange or unpleasant sensation in the skin, altered taste, motion-induced dizziness, low blood pressure, severe respiratory problems, nasal bleeding, nasal ulcer, runny nose, constipation, mouth inflammation, dry mouth, skin discomfort, skin itching, fever.
Unknown(frequency cannot be estimated from available data):
Allergic reaction, falls, diarrhea, seizures (epileptic crisis), loss of consciousness, inflammation of arms or legs, seeing or hearing things that are not real (hallucinations), delirium (symptoms may include a combination of agitation, restlessness, disorientation, confusion, fear, seeing or hearing things that do not exist, sleep disorders, nightmares), pharmacological tolerance, drug dependence (addiction), drug abuse (see section 2), fatigue, general malaise, withdrawal syndrome (which may manifest with the appearance of the following adverse effects: nausea, vomiting, diarrhea, anxiety, chills, tremors, and sweating), breathing difficulties.
Cases have been observed in patients who have developed a perforation in the nasal septum – the structure that separates the nostrils.
Prolonged treatment with fentanyl during pregnancy may cause withdrawal symptoms in the newborn, which can be potentially fatal (see section 2).
You should inform your doctor if you suffer from repeated episodes of nasal bleeding or nose discomfort.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Instanyl
The analgesic contained in Instanyl is very potent and can be fatal in children. Instanyl must be kept out of reach and sight of children.
Keep this medicine in a safe and protected place, where others cannot access it. This medicine can cause serious harm and even be fatal for people who use it accidentally or intentionally when it has not been prescribed for them.
Do not use Instanyl after the expiration date that appears on the box and on the single-dose container after CAD or EXP. The expiration date is the last day of the indicated month.
Store below 30°C. Keep the blister in the outer packaging. Store in a vertical position.
Instanyl containers can harm other people, especially children. Medicines should not be thrown down the drain or into the trash. Any unused single-dose container should be returned according to local regulations or returned to the pharmacy in the child-resistant blister pack. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Instanyl
The active ingredient is fentanyl citrate. The content is:
50 micrograms: 1 dose (100 microliters) contains fentanyl citrate equivalent to 50 micrograms of fentanyl.
100 micrograms: 1 dose (100 microliters) contains fentanyl citrate equivalent to 100 micrograms of fentanyl.
200 micrograms: 1 dose (100 microliters) contains fentanyl citrate equivalent to 200 micrograms of fentanyl.
The other components are sodium dihydrogen phosphate dihydrate, disodium phosphate dihydrate, and water for injectable preparations.
Appearance of Instanyl and Package Contents
Instanyl is a nasal spray solution in a single-dose container. The solution is clear and colorless.
The single-dose container contains 1 dose of Instanyl and is dispensed in a child-resistant blister pack. Instanyl is available in different package sizes of 2, 6, 8, and 10 single-dose containers.
Not all package sizes may be marketed.
The labeling of the three Instanyl concentrations is differentiated by color:
For 50 micrograms, the labeling is orange.
For 100 micrograms, the labeling is purple.
For 200 micrograms, the labeling is greenish-blue.
Marketing Authorization Holder
Istituto Gentili S.r.l.
Via San Giuseppe Cottolengo 15
20143 Milano
Italy
Manufacturer
Curida AS
Solbærvegen 5
NO-2409 Elverum
Norway
Date of Last Revision of this Prospectus
August 2024
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
INSTRUCTIONS FOR USE OF THE INSTANYL SINGLE-DOSE NASAL SPRAY
Read the following instructions carefully to learn how to use the Instanyl single-dose nasal spray:
- Each single-dose container is sealed in a child-resistant blister pack. Do not open the blister pack until you are ready to use the spray. Each single-dose container contains a single dose of Instanyl, so do not test it before use.
- To open, cut with scissors along the line (on the scissors symbol) on the blister pack. Hold the edge of the foil, peel off the foil, and remove the nasal spray.
- Blow your nose if it is blocked or if you have a cold.
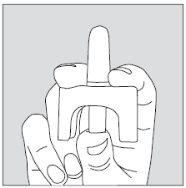
- Carefully hold the single-dose container by placing your thumb on the bottom of the actuator and your index and middle fingers on either side of the top of the spray (see drawing). Do not press the actuator yet.
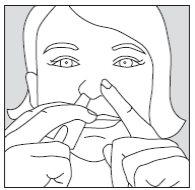
- Close one nostril by pressing your index finger against the side of your nose and insert the spray into the other nostril (approximately 1 cm). It does not matter which nostril you use. If after 10 minutes you need to use a second dose to achieve sufficient pain relief, this dose should be administered in the other nostril.
- Keep your head upright.
- To release the dose, firmly press the actuator upwards with your thumb while carefully inhaling through your nose. Then remove the nasal spray from your nose. You may not feel the dose in your nose, but you will have received it when you press the actuator.
Your single-dose container is now empty.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INSTANYL 200 micrograms NASAL SPRAY SOLUTION IN SINGLE-DOSE CONTAINERDosage form: TRANSDERMAL PATCH, 12 MCG/HActive substance: fentanylManufacturer: Aristo Pharma Iberia S.L.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1200 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1600 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription required
Online doctors for INSTANYL 200 micrograms NASAL SPRAY SOLUTION IN SINGLE-DOSE CONTAINER
Discuss questions about INSTANYL 200 micrograms NASAL SPRAY SOLUTION IN SINGLE-DOSE CONTAINER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















