
INNOHEP 12,000 IU Anti-Xa/0.6 mL Pre-filled Syringe Solution for Injection

How to use INNOHEP 12,000 IU Anti-Xa/0.6 mL Pre-filled Syringe Solution for Injection
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
innohep 12,000 IU anti-Xa/0.6 ml injectable solution in pre-filled syringes
(20,000 IU anti-Xa/ml)
tinzaparin sodium
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again. If you have any questions, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet.
- In the text of this leaflet, the term innohep will be used to refer to innohep 12,000 IU anti-Xa/0.6 ml injectable solution in pre-filled syringes.
Contents of the leaflet
- What is innohep and what is it used for
- What you need to know before using innohep
- How to use innohep
- Possible side effects
- Storage of innohep
- Package contents and additional information
1. What is innohep and what is it used for
innohep is a medicine that inhibits the natural ability of blood to form clots (coagulate).
- innohep is used to treat blood clots and prevent the formation of more clots in adults.
You should consult a doctor if it worsens or does not improve.
2. What you need to know before using innohep
Do not use innohep
- if you are allergic to tinzaparin sodium or any of the other components of this medicine (listed in section 6).
- if you have or have had heparin-induced thrombocytopenia.
- if you have a severe bleeding disorder (e.g., in the brain, spinal cord, eyes, or stomach).
- if you have a severe infection in the heart (septic endocarditis).
- if you are scheduled for spinal or epidural anesthesia or a lumbar puncture.
Warnings and precautions
Consult your doctor or nurse before starting to use innohep
- if you know you have a tendency to bleed.
- if you are being treated with other medicines by intramuscular route.
- if you have a low platelet count in your blood.
- if you have high potassium levels in your blood (hyperkalemia).
- if you have a prosthetic heart valve.
- if you have kidney problems.
Children and adolescents
innohep is not indicated for use in children and adolescents.
Using innohep with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine. Some medicines may interact with the effect of innohep.
Tell your doctor if you are using any of the following medicines, as you may bleed more easily:
- Medicines for the treatment of inflammation and pain, especially non-steroidal anti-inflammatory drugs (NSAIDs), such as acetylsalicylic acid.
- Medicines used to dissolve blood clots (thrombolytic agents).
- Medicines that block the action of vitamin K (vitamin K antagonists).
- Activated protein C.
- Direct inhibitors of factor Xa and IIa.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
innohep in pre-filled syringes can be used during all trimesters of pregnancy.
If epidural anesthesia is necessary, you should inform your doctor that you are using innohep.
Tell your doctor or nurse if you have a prosthetic heart valve.
Driving and using machines
innohep does not affect the ability to drive or use machines.
innohep contains sodium metabisulfite
- As it contains sodium metabisulfite as an excipient, innohep may rarely cause severe allergic reactions and bronchospasm.
- This medicine contains 40 mg of sodium (main component of table/cooking salt) per ml. This is equivalent to 2% of the maximum recommended daily sodium intake for an adult.
3. How to use innohep
Follow exactly the administration instructions of this medicine indicated by your doctor or nurse. If in doubt, consult your doctor or nurse again.
During treatment, your doctor may request that you undergo routine blood tests to assess the effect of innohep.
innohep should be injected under the skin (subcutaneous injection). Administration by intramuscular injection of other medicines should be avoided during treatment with innohep, due to the risk of hematoma formation.
The recommended dose is:
175 IU anti-Xa per kg of body weight administered once a day.
Your doctor will indicate the appropriate dose for you.
Treatment should be administered once a day for 6 days, and may be extended up to 6 months. The need to extend treatment for more than 6 months should be evaluated by your doctor. Your doctor will indicate the duration of your treatment.
Instructions on how to inject innohep:
- Wash your hands carefully before injecting this medicine. Clean the skin around the injection site with an alcohol swab and let it dry without rubbing.
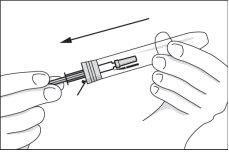
- Open the syringe container by lifting the colored cap all the way back, and remove the syringe.
Inspect the contents of the syringe before use. If you observe turbidity or a precipitate in the medicine, do not use it and take another syringe. The medicine may have a yellowish color during storage, but it can be used as long as the solution is clear and the expiration date has not been exceeded. Each syringe can only be used once.
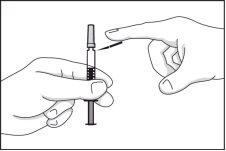
- Fold the safety device downwards, separating it from the needle protector.
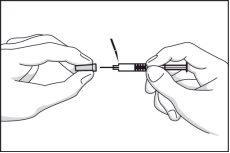
- Remove the cap that protects the needle without twisting it. Adjust the syringe contents to the prescribed dose by your doctor. Eliminate excess volume by pressing the plunger in a vertical position. Do not pull the plunger back or eliminate the air bubble contained in the syringe. If the air bubble is not correctly positioned next to the plunger, gently tap the syringe until the air bubble is in place.
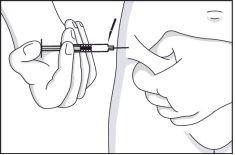
- Take a skin fold, without pressing, between the thumb and index finger of one hand, and with the other hand, insert the needle vertically and slowly into the skin fold, i.e., at a right angle to the skin.
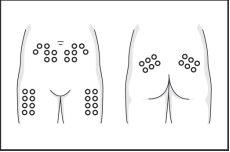
- Slowly inject the necessary dose into the fatty tissue, for example, the skin of the abdominal area, the front of the thighs, the lower back, or the back of the arms. Wait a few seconds to allow the solution to distribute before removing the needle and releasing the skin fold.
- Clean the area with a swab if bleeding occurs. Choose a different injection site for the next time (e.g., alternating between the left and right side of the abdomen).
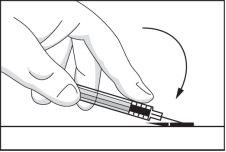
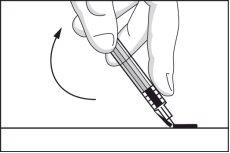
- Fold the safety device back to its original position so that it is parallel to the needle. Then, with the safety device flat on a rigid surface, press down until the needle is locked into the device.
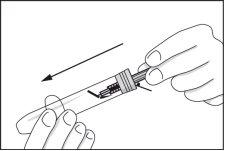
- The used syringe, with the needle facing down, can be inserted into the syringe container or a sharps container. In this way, the syringe is safe, and the container with the syringe or the sharps container can be returned to a hospital or pharmacist for destruction.
If you use more innohep than you should
If you are treated with more innohep than you need, bleeding may occur. Consult your doctor or nurse immediately if you do not feel well or think you have been given too high a dose of innohep.
If you forget to use innohep
Do not inject a double dose to make up for the forgotten dose. If you have forgotten to administer more than one dose, consult your doctor as soon as possible.
If you stop using innohep
If you stop using innohep, the effect of making the blood more fluid will cease. Do not stop using innohep without consulting your doctor or nurse.
If you have any other questions about using this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, innohep can cause side effects, although not everyone gets them.
Severe side effects:
Rarely, severe side effects have been observed that require immediate medical attention during treatment with innohep. If you experience any of the following symptoms, consult your doctor or go to the nearest hospital for emergency treatment.
- Severe allergic reaction. Symptoms include sudden appearance of a severe skin rash, swelling of the throat, face, lips, or mouth, difficulty breathing.
- Severe bleeding. Symptoms include red or brown urine, black stools, unusual bruising (very painful, extensive, or dark), and any bleeding that does not stop.
The following side effects have been observed with the administration of innohep:
Common: may affect up to 1 in 10 people
- Bleeding: May lead to complications, such as anemia (low red blood cell count) or bruising.
- Reactions at the injection site (including bruising, bleeding, pain, itching, redness, inflammation, and hardening of the injection site).
Uncommon: may affect up to 1 in 100 people
- Thrombocytopenia (decrease in platelet count in the blood).
- Hypersensitivity (allergic reaction).
- Bruising and skin discoloration.
- Elevated liver enzyme levels.
- Dermatitis (skin inflammation).
- Rash and itching.
Rare: may affect up to 1 in 1,000 people
- Heparin-induced thrombocytopenia (decrease in platelet count in the blood due to heparin treatment).
- Thrombocytosis (increase in platelet count in the blood).
- Angioedema (swelling of the face, lips, and tongue).
- Anaphylactic reaction (see above "Severe allergic reaction").
- Hyperkalemia (elevated potassium levels in the blood).
- Toxic skin rash.
- Necrosis of the skin (death of skin tissue).
- Hives.
- Osteoporosis, observed in long-term treatments.
- Priapism (prolonged, often painful erection, without prior sexual stimulation).
Pediatric population
Limited information is available from a clinical trial and post-marketing data, indicating that the profile of adverse reactions in children and adolescents is comparable to that observed in adults.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of innohep
- Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
- No special storage conditions are required.
- Do not use this medicine if you observe turbidity or a precipitate in the syringe.
- The solution may have a yellowish color during storage, but this does not affect the quality of the product, which is still safe for use.
- Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package contents and additional information
Composition of innohep
- The active ingredient is tinzaparin sodium. Each ml of injectable solution contains 20,000 IU anti-Xa of tinzaparin sodium.
- The other ingredients are sodium metabisulfite (E 223), sodium hydroxide, and water for injections.
Appearance and packaging of the product
Injectable solution.
Transparent glass syringes containing a clear or pale yellow solution, free from turbidity and deposit when the syringe is left to stand.
Package sizes:
- 0.6 ml (12,000 IU anti-Xa), packs of 2, 6, 10, 30, 50, and 100 syringes.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
LEO Pharma A/S
Industriparken 55,
DK-2750 Ballerup
Denmark
Manufacturer:
Laboratoires LEO S.A.
39 Route de Chartres,
FR-28500 Vernouillet Cedex
France
Date of last revision of this leaflet:December 2019.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price121.76 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INNOHEP 12,000 IU Anti-Xa/0.6 mL Pre-filled Syringe Solution for InjectionDosage form: INJECTABLE, 10000 IUActive substance: tinzaparinManufacturer: Leo Pharma A/SPrescription requiredDosage form: INJECTABLE, 14,000 IUActive substance: tinzaparinManufacturer: Leo Pharma A/SPrescription requiredDosage form: INJECTABLE, 16,000 IU anti-XaActive substance: tinzaparinManufacturer: Leo Pharma A/SPrescription required
Online doctors for INNOHEP 12,000 IU Anti-Xa/0.6 mL Pre-filled Syringe Solution for Injection
Discuss questions about INNOHEP 12,000 IU Anti-Xa/0.6 mL Pre-filled Syringe Solution for Injection, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















