
INIXA 12,000 IU (120 mg)/0.8 mL Injectable Solution

How to use INIXA 12,000 IU (120 mg)/0.8 mL Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Inhixa 12,000 IU (120 mg)/0.8 ml Solution for Injection
Inhixa 15,000 IU (150 mg)/1 ml Solution for Injection
Sodium Enoxaparin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Inhixa and what is it used for
- What you need to know before you use Inhixa
- How to use Inhixa
- Possible side effects
- Storing Inhixa
- Package Contents and Additional Information
1. What is Inhixa and what is it used for
Inhixa contains the active substance sodium enoxaparin, which is a low molecular weight heparin (LMWH).
Inhixa works in two ways:
- Preventing existing blood clots from getting bigger. This helps your body to break them down and stop them from causing harm.
- Stopping new blood clots from forming.
Inhixa can be used to:
- treat blood clots
- prevent blood clots from forming in the following situations:
- before and after surgery
- when you have an acute illness and will be immobile for a period
- if you have had blood clots due to cancer, to prevent new clots from forming
- if you have unstable angina (a condition where not enough blood reaches the heart)
- after a heart attack
- prevent blood clots from forming in the tubes of a dialysis machine (used in people with severe kidney problems).
2. What you need to know before you use Inhixa
Do not use Inhixa
- If you are allergic to sodium enoxaparin or any of the other ingredients of this medicine (listed in section 6). Signs of an allergic reaction include: rash, problems swallowing or breathing, swelling of the lips, face, throat, or tongue.
- If you are allergic to heparin or other low molecular weight heparins such as nadroparin, tinzaparin, or dalteparin.
- If you have had a reaction to heparin that caused a severe decrease in the number of cells involved in blood clotting (platelets) – this reaction is called heparin-induced thrombocytopenia – in the last 100 days or if you have antibodies against enoxaparin in your blood.
- If you are bleeding heavily or have a high risk of bleeding (such as a stomach ulcer, recent eye or brain surgery), including a recent hemorrhagic stroke.
- If you are using Inhixa to treat blood clots and will be receiving spinal or epidural anesthesia or a lumbar puncture within 24 hours.
Warnings and Precautions
Inhixa should not be exchanged with other medicines that belong to the group of low molecular weight heparins. This is because they are not exactly the same and do not have the same activity or instructions for use.
Consult your doctor or pharmacist before starting to use Inhixa if:
- you have ever had a reaction to heparin that caused a severe decrease in the number of platelets
- you will be receiving spinal/epidural anesthesia or a lumbar puncture (see "Surgical Operations and Anesthesia"): a delay should be observed between Inhixa and the use of this procedure
- you have had a heart valve implanted
- you have endocarditis (an infection of the inner lining of the heart)
- you have a history of stomach ulcers
- you have recently had a stroke
- you have high blood pressure
- you have diabetes or problems with the blood vessels in the eyes caused by diabetes (called diabetic retinopathy)
- you have recently had eye or brain surgery
- you are an elderly person (over 65 years old) and especially if you are over 75 years old
- you have kidney problems
- you have liver problems
- you are underweight or overweight
- you have high levels of potassium in your blood (which could be checked with a blood test)
- you are currently using medicines that affect bleeding (see below - Using Inhixa with other medicines)
You may need to have a blood test before starting to use this medicine and while you are using it; this is to check the level of cells involved in blood clotting (platelets) and the levels of potassium in your blood.
Children and Adolescents
The safety and efficacy of Inhixa in children and adolescents have not been evaluated.
Using Inhixa with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
- Warfarin - another anticoagulant medicine used to reduce blood clotting
- Acetylsalicylic acid (also known as aspirin or AAS), clopidogrel, or other medicines used to prevent blood clots from forming (see also section 3, "Change of Anticoagulant Treatment")
- Dextran injection - used as a blood substitute
- Ibuprofen, diclofenac, ketorolac, and other medicines known as non-steroidal anti-inflammatory drugs used to treat pain and inflammation in arthritis and other diseases
- Prednisolone, dexamethasone, and other medicines used to treat asthma, rheumatoid arthritis, and other diseases
- Medicines that increase the level of potassium in your blood, such as potassium salts, diuretics, and some medicines for heart problems.
Surgical Operations and Anesthesia
If you are going to have a lumbar puncture or undergo surgery where spinal or epidural anesthesia will be used, tell your doctor that you are using Inhixa. See "Using Inhixa with other medicines". Also, tell your doctor if you have any problems with your spine or if you have had spine surgery.
Pregnancy and Breastfeeding
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
If you are pregnant and have a mechanical heart valve, you may have a higher risk of blood clots. Your doctor will discuss this with you.
If you are breastfeeding or plan to breastfeed, you should consult your doctor before using this medicine.
Driving and Using Machines
Inhixa does not affect your ability to drive or use machines.
Traceability
It is important to keep a record of the batch number of your Inhixa. Therefore, each time you receive a new pack of Inhixa, note the date and batch number (which is on the pack after "Batch") and keep this information in a safe place.
Inhixa contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to use Inhixa
Follow your doctor's or pharmacist's administration instructions for this medication exactly. If in doubt, consult your doctor or pharmacist again.
Using the medication
- Normally, your doctor or nurse will administer Inhixa to you. This is because it must be administered via injection.
- When you return home, you may need to continue using Inhixa and administer it yourself (see the instructions on how to do this).
- Inhixa is usually administered by subcutaneous injection (under the skin).
- Inhixa can be administered intravenously (into your veins) after certain types of heart attacks and surgical operations.
- Inhixa can be added to the tube coming out of your body (arterial line) at the start of a dialysis session.
Do not administer Inhixa intramuscularly (into a muscle).
How much will you be given
- Your doctor will decide the amount of Inhixa you will be given. The dose will depend on the reason it is being used.
- If you have kidney problems, you may be given a smaller amount of Inhixa.
1) Treatment of blood clot formation:
- The usual dose is 150 IU (1.5 mg) per kilogram of body weight once a day or 100 IU (1 mg) per kilogram of body weight twice a day.
- Your doctor will decide how long you will receive Inhixa.
- Prevention of blood clot formation in the following situations:
- Surgery or periods of limited mobility due to illness
- The dose will depend on your likelihood of developing a clot. You will be given 2,000 IU (20 mg) or 4,000 IU (40 mg) of Inhixa per day.
- If you are going to have surgery, you will usually be given the first injection 2 or 12 hours before the operation.
- If you have reduced mobility due to illness, you will usually be given 4,000 IU (40 mg) of Inhixa per day.
- Your doctor will decide how long you will receive Inhixa.
- After having a heart attack
Inhixa can be used in 2 different types of heart attacks, known as STEMI (ST-elevation myocardial infarction) or non-STEMI. The amount of Inhixa you are given will depend on your age and the type of heart attack you had.
Non-STEMI heart attack:
- The usual dose is 100 IU (1 mg) per kilogram of body weight every 12 hours.
- Usually, your doctor will tell you to also take aspirin (acetylsalicylic acid).
- Your doctor will decide how long you will receive Inhixa.
STEMI heart attack if you are under 75 years old:
- You will be given an initial intravenous injection of 3,000 IU (30 mg) of Inhixa.
- At the same time, you will be given a subcutaneous injection of Inhixa. The usual dose is 100 IU (1 mg) per kilogram of body weight every 12 hours.
- Usually, your doctor will tell you to also take aspirin (acetylsalicylic acid).
- Your doctor will decide how long you will receive Inhixa.
STEMI heart attack if you are 75 years old or older:
- The usual dose is 75 IU (0.75 mg) per kilogram of body weight every 12 hours.
- The maximum amount of Inhixa given in the first two injections is 7,500 IU (75 mg).
- Your doctor will decide how long you will receive Inhixa.
For patients undergoing percutaneous coronary intervention (PCI):
Depending on when you last received an injection of Inhixa, your doctor may decide to give you an additional dose of Inhixa before a PCI procedure. This would be given intravenously.
- Prevention of blood clot formation in the dialysis machine tubes
- The usual dose is 100 IU (1 mg) per kilogram of body weight.
- Inhixa is added to the tube coming out of your body (arterial line) at the start of the dialysis session. This amount is usually sufficient for a 4-hour session. However, your doctor may give you another injection of 50 IU to 100 IU/kg (0.5 to 1 mg/kg) per kilogram of body weight if necessary.
Self-administering an Inhixa injection with a pre-filled syringe without a needle guard
If you can administer this medication yourself, your doctor or nurse will show you how to do it. Do not attempt to inject yourself if you have not been taught how to do it. If you are unsure what to do, consult your doctor or nurse immediately.
Before injecting Inhixa
- Check the expiration date of the medication. If it has expired, do not use it.
- Check if the syringe is not damaged and the liquid inside is transparent. If not, use another syringe.
- Do not use this medication if you notice any change in its appearance.
- Check the amount to be injected.
- Review if the last injection caused redness, skin color change, swelling, suppuration, or if it still hurts. If so, talk to your doctor or nurse.
- Decide on the area where you will inject the medication. Alternate, each time you inject, the right side of the abdomen (belly) with the left. This medication should be injected just under the skin of the abdomen, but not too close to the navel or any scar (at least 5 cm away from them).
- The pre-filled syringe is for single use only.
Instructions for self-injecting Inhixa
- Sit or lie down in a comfortable and relaxed position. Make sure you can see the area where you will inject. The most suitable place is on a couch, a reclining chair, or in a bed with pillows to support yourself.
- Choose an area on the right or left side of the belly. It should be more than 5 cm away from the navel and towards the sides.
Remember.Do not inject in the 5 cm around the navel or scars or hematomas that you may have. Inject in the opposite area to the one you injected the last time (alternating the right side of the belly with the left).
- Take the plastic blister pack containing the pre-filled syringe out of the box. Open the blister pack and remove the pre-filled syringe.
- Carefully remove the needle cap from the syringe by pulling it. The syringe is pre-filled and ready for use.
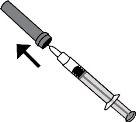
Do notpress the plunger before injecting. Once you have removed the cap, do not touch anything with the needle. This will ensure that the needle remains clean (sterile).
- Hold the syringe with the hand you write with (like a pencil) and, with the other hand, gently pinch the area of the abdomen between your index finger and thumb to form a skin fold.
Make sure to hold the skin fold throughout the injection.
- Hold the syringe so that the needle points downwards (vertically at a 90-degree angle). Insert the entire needle into the skin fold.
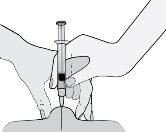
- Press the plunger with your thumb. This will inject the medication into the abdominal fatty tissue. Make sure to hold the skin fold throughout the injection.
- Remove the needle by pulling it straight out.
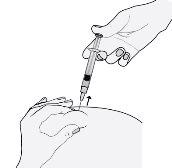
To avoid a hematoma, do not rub the injection area after injecting.
- Place the used syringe in the sharps container. Close the container lid tightly and keep it out of the reach of children.
When the container is full, dispose of it as your doctor or pharmacist has instructed. Do not throw it in the trash.
Self-administering an Inhixa injection with a pre-filled syringe with a needle guard
Your pre-filled syringe includes a needle guard to protect you from a needle stick injury.
If you can administer this medication yourself, your doctor or nurse will show you how to do it. Do not attempt to inject yourself if you have not been taught how to do it. If you are unsure what to do, consult your doctor or nurse immediately.
Before injecting Inhixa
- Check the expiration date of the medication. If it has expired, do not use it.
- Check if the syringe is not damaged and the liquid inside is transparent. If not, use another syringe.
- Do not use this medication if you notice any change in its appearance.
- Check the amount to be injected.
- Review if the last injection caused redness, skin color change, swelling, suppuration, or if it still hurts. If so, talk to your doctor or nurse.
- Decide on the area where you will inject the medication. Alternate, each time you inject, the right side of the abdomen (belly) with the left. This medication should be injected just under the skin of the abdomen, but not too close to the navel or any scar (at least 5 cm away from them).
- The pre-filled syringe is for single use only.
Instructions for self-injecting Inhixa
- Sit or lie down in a comfortable and relaxed position. Make sure you can see the area where you will inject. The most suitable place is on a couch, a reclining chair, or in a bed with pillows to support yourself.
- Choose an area on the right or left side of the belly. It should be more than 5 cm away from the navel and towards the sides.
Remember.Do not inject in the 5 cm around the navel or scars or hematomas that you may have. Inject in the opposite area to the one you injected the last time (alternating the right side of the belly with the left).
- Take the plastic blister pack containing the pre-filled syringe out of the box. Open the blister pack and remove the pre-filled syringe.
- Carefully remove the needle cap from the syringe by pulling it. The syringe is pre-filled and ready for use.

Do notpress the plunger before injecting. Once you have removed the cap, do not touch anything with the needle. This will ensure that the needle remains clean (sterile).
- Hold the syringe with the hand you write with (like a pencil) and, with the other hand, gently pinch the area of the abdomen between your index finger and thumb to form a skin fold.
Make sure to hold the skin fold throughout the injection.
- Hold the syringe so that the needle points downwards (vertically at a 90-degree angle). Insert the entire needle into the skin fold.
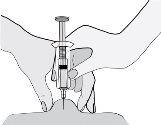
- Press the plunger with your thumb. This will inject the medication into the abdominal fatty tissue. Make sure to hold the skin fold throughout the injection.
- Remove the needle by pulling it straight out. Do not release the plunger!
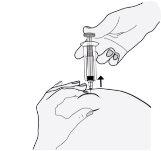
To avoid a hematoma, do not rub the injection area after injecting.
- Release the plunger and allow the syringe to move up until the entire needle is guarded and locked in place.

- Place the used syringe in the sharps container. Close the container lid tightly and keep it out of the reach of children.
When the container is full, dispose of it as your doctor or pharmacist has instructed. Do not throw it in the trash.
Changing anticoagulant treatment
- Changing from Inhixa to vitamin K antagonist medications (e.g., warfarin)
Your doctor will request a blood test to determine a parameter called INR and will tell you when to stop taking Inhixa.
- Changing from vitamin K antagonist medications (e.g., warfarin) to Inhixa
Stop taking the vitamin K antagonist. Your doctor will request a blood test to determine a parameter called INR and will tell you when to start using Inhixa.
- Changing from Inhixa to direct oral anticoagulants (e.g., apixaban, dabigatran, edoxaban, rivaroxaban)
Stop taking Inhixa. Start taking the direct oral anticoagulant 0-2 hours before the next scheduled Inhixa injection, and then continue as usual.
- Changing from direct oral anticoagulant to Inhixa
Stop taking the direct oral anticoagulant. Do not start Inhixa treatment until 12 hours after the last dose of the direct oral anticoagulant.
If you use more Inhixa than you should
If you think you have used too much or too little Inhixa, inform your doctor, nurse, or pharmacist immediately, even if you do not have any symptoms. If a child accidentally injects or swallows Inhixa, take them to the hospital emergency department immediately.
If you forget to use Inhixa
If you forget to administer a dose, do it as soon as you remember. Do not use a double dose on the same day to make up for missed doses. To ensure you do not miss any doses, it may be helpful to use a diary.
If you stop using Inhixa
If you have any other questions about using this medication, ask your doctor, pharmacist, or nurse. It is essential that you continue to receive Inhixa until your doctor decides to stop the treatment. If you stop using it, a blood clot may form, which can be very dangerous.
4. Possible Adverse Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Like other anticoagulant medicines (medicines to reduce blood clots), Inhixa could cause bleeding, which could potentially put your life in danger. In some cases, the bleeding may not be apparent.
If you notice any episode of bleeding that does not stop by itself or if you notice signs of excessive bleeding (unusual weakness, fatigue, paleness, dizziness, headache, or unexplained swelling), consult your doctor immediately.
Your doctor may decide to keep you under close observation or change your medication.
Stop treatment with Inhixa and inform your doctor or nurse immediately if you experience any sign of a severe allergic reaction (such as difficulty breathing, swelling of the lips, mouth, throat, or eyes).
Stop treatment with enoxaparina and inform your doctor or nurse immediately if you experience any of the following symptoms:
- A widespread, red, and scaly rash, with bumps under the skin and blisters, accompanied by fever. The symptoms usually appear at the start of treatment (acute generalized exanthematous pustulosis).
You should inform your doctor immediately
- If you present any sign of a blood vessel blockage due to a blood clot, such as:
cramp-like pain, redness, heat, or swelling in one of your legs – which are symptoms of deep vein thrombosis
difficulty breathing, chest pain, fainting, or coughing up blood – which are symptoms of pulmonary embolism
- If you have a painful skin rash with dark red spots under the skin that do not disappear when pressed.
Your doctor may request that you have a blood test to check the number of platelets.
General List of Possible Adverse Effects:
Very common (may affect more than 1 in 10 people)
- Bleeding.
- Increased liver enzymes.
Common (may affect up to 1 in 10 people)
- If bruises appear more frequently than usual. This could be due to a blood problem due to a low number of platelets.
- Pink patches on the skin. They appear more frequently in the area where you have been injected with Inhixa.
- Skin rash (hives, urticaria).
- Redness and itching of the skin.
- Bruising or pain at the injection site.
- Decrease in the number of red blood cells in the blood.
- Increase in the number of platelets in the blood.
- Headache.
Uncommon (may affect up to 1 in 100 people)
- Sudden severe headache. This could be a sign of bleeding in the brain.
- Feeling of sensitivity to palpation and swelling of the stomach. It could be indicative of gastric bleeding.
- Large, irregular red marks on the skin, with or without blisters.
- Skin irritation (local irritation).
- Yellowing of the skin or eyes, and darkening of the urine color. These could be signs of a liver problem.
Rare (may affect up to 1 in 1,000 people)
- Severe allergic reaction. The signs of this reaction could include: skin rash, swallowing or breathing problems, swelling of the lips, face, throat, or tongue.
- Increased potassium in the blood. This is more likely to happen in people with kidney problems or diabetes. Your doctor can check this by doing a blood test.
- Increased number of white blood cells called eosinophils in the blood. Your doctor can check this by doing a blood test.
- Hair loss.
- Osteoporosis (a disease in which bones can fracture more easily).
- Tingling, numbness, and weakness in the muscles (especially in the lower part of the body) when you have had a lumbar puncture or spinal anesthesia.
- Loss of bladder or bowel control (so that you cannot control your needs).
- Hardening or nodule at the injection site.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Inhixa
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the label and carton. The expiration date is the last day of the month indicated.
Store below 25°C. Do not freeze.
The solution must be used within 8 hours after its dilution.
Do not use this medicine if you notice any visible change in the appearance of the solution.
The pre-filled syringes of Inhixa are for single use only. Discard the unused contents of the medicine.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Inhixa
- The active ingredient is enoxaparin sodium.
Each milliliter contains 15,000 IU (150 mg) of enoxaparin sodium.
Each pre-filled syringe of 0.8 ml contains 12,000 IU (120 mg) of enoxaparin sodium.
Each pre-filled syringe of 1 ml contains 15,000 IU (150 mg) of enoxaparin sodium.
- The other components are water for injectable preparations.
Appearance of the Product and Package Contents
Inhixa 12,000 IU (120 mg)/0.8 ml is 0.8 ml of solution contained in:
- a transparent, colorless, neutral glass syringe of type I, with a fixed needle and needle protector closed with a chlorobutyl rubber stopper and a purple polypropylene plunger. The syringe can be equipped with an additional needle protector.
This medicine is presented in packs of:
- 2, 10, and 30 pre-filled syringes
- 10 and 30 pre-filled syringes with needle protector
Inhixa 15,000 IU (150 mg)/1 ml is 1 ml of solution contained in:
- a transparent, colorless, neutral glass syringe of type I, with a fixed needle and needle protector closed with a chlorobutyl rubber stopper and a dark blue polypropylene plunger. The syringe can be equipped with an additional needle protector.
This medicine is presented in packs of:
- 2, 10, and 30 pre-filled syringes
- 10 and 30 pre-filled syringes with needle protector
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Techdow Pharma Netherlands B.V.
Strawinskylaan 1143, Toren C-11
1077XX Amsterdam
Netherlands
Manufacturer
SciencePharma spólka z ograniczona odpowiedzialnoscia
Chelmska 30/34
00-725 Varsovia
Poland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Techdow Pharma Netherlands B.V.+31 (0)76 531 5388 | Lietuva Techdow Pharma Netherlands B.V. +37125892152 |
| Luxembourg/Luxemburg Techdow Pharma Netherlands B.V. +49 (0)30 220 13 6906 |
Ceská republika Techdow Pharma Netherlands B.V. +420255790502 | Magyarország Techdow Pharma Netherlands B.V. +3618001930 |
Danmark Techdow Pharma Netherlands B.V. +4578774377 | Malta Mint Health Ltd +441483928995 |
Deutschland Mitvertrieb: Techdow Pharma Germany GmbH Potsdamer Platz 1, 10785 Berlin +49 (0)30 98 321 31 00 | Nederland Techdow Pharma Netherlands B.V. +31208081112 |
Eesti Techdow Pharma Netherlands B.V. +37125892152 | Norge Techdow Pharma Netherlands B.V. +4721569855 |
Ελλáδα Techdow Pharma Netherlands B.V. +49 (0)30 220 13 6906 | Österreich Techdow Pharma Netherlands B.V. +43720230772 |
España TECHDOW PHARMA SPAIN, S.L. Tel: +34 91 123 21 16 | Polska Techdow Pharma Netherlands B.V. +49 (0)30 220 13 6906 |
France Viatris Santé +33 4 37 25 75 00 | Portugal Laboratórios Atral, S.A. +351308801067 |
Hrvatska Techdow Pharma Netherlands B.V. +385 17776255 Ireland Techdow Pharma England Ltd +441483928995 | România Techdow Pharma Netherlands B.V. +49 (0)30 220 13 6906 Slovenija Techdow Pharma Netherlands B.V. +49 (0)30 220 13 6906 |
Ísland Techdow Pharma Netherlands B.V. +49 (0)30 220 13 6906 | Slovenská republika Techdow Pharma Netherlands B.V. +421233331071 |
Italia Techdow Pharma Italy S.R.L. Tel: +39 0256569157 | Suomi/Finland Techdow Pharma Netherlands B.V. +358942733040 |
Κúπρος MA Pharmaceuticals Trading Ltd +357 25 587112 | Sverige Techdow Pharma Netherlands B.V. +46184445720 |
Latvija Techdow Pharma Netherlands B.V. +37125892152 | United Kingdom(Northern Ireland) Techdow Pharma Netherlands B.V + 44 28 9279 2030 |
Date ofthe last revision of this prospectus:
Other sources of information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price86.05 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INIXA 12,000 IU (120 mg)/0.8 mL Injectable SolutionDosage form: INJECTABLE, 100 mg (10000 IU) enoxaparin sodium/mlActive substance: enoxaparinManufacturer: Sanofi Aventis S.A.Prescription requiredDosage form: INJECTABLE, 120 mg (12000 IU) /0.8 mlActive substance: enoxaparinManufacturer: Sanofi Aventis S.A.Prescription requiredDosage form: INJECTABLE, 150 mg (15000 IU) /1 mlActive substance: enoxaparinManufacturer: Sanofi Aventis S.A.Prescription required
Online doctors for INIXA 12,000 IU (120 mg)/0.8 mL Injectable Solution
Discuss questions about INIXA 12,000 IU (120 mg)/0.8 mL Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















