
INCRUSE ELLIPTA 55 mcg INHALATION POWDER, SINGLE-DOSE

How to use INCRUSE ELLIPTA 55 mcg INHALATION POWDER, SINGLE-DOSE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Incruse Ellipta 55microgramspowder forinhalation (single dose)
umeclidinium (umeclidinium)
Read this package leaflet carefully before you start using this medicine, because it containsimportant information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only, do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Incruse Ellipta and what is it used for
- What you need to know before you start using Incruse Ellipta
- How to use Incruse Ellipta
- Possible side effects
- Storage of Incruse Ellipta
- Contents of the pack and further information
Step-by-step instructions for use
1. What is Incruse Ellipta and what is it used for
What is Incruse Ellipta
Incruse Ellipta contains the active substance umeclidinium (as bromide), which belongs to a group of medicines called bronchodilators.
What is Incruse Ellipta used for
This medicine is used to treat chronic obstructive pulmonary disease (COPD)(COPD)in adults. COPD is a long-term disease that gradually worsens, and the airways and air sacs in the lungs become obstructed or damaged, making it difficult to breathe. This difficulty breathing is added to the contraction of the muscles surrounding the airways, making them narrower and more difficult for air to flow through.
This medicine prevents the contraction of these muscles in the lungs, making it easier for air to enter and leave the lungs. When used regularly, it helps control breathing difficulties and reduces the effects of COPD on daily life.
Incruse Ellipta should not be used to relieve a sudden attack of shortness of breath or wheezing.
If you have this type of attack, you should use a fast-acting "rescue" inhaler (such as salbutamol). If you do not have a fast-acting inhaler, contact your doctor.
2. What you need to know before you start using Incruse Ellipta
Do not use Incruse Ellipta
- if you are allergic to umeclidinium or any of the other ingredients of this medicine (listed in section 6).
If you think the above applies to you, do not usethis medicine until you have consulted your doctor.
Warnings and precautions
Consult your doctor before starting to use Incruse Ellipta:
- if you have asthma(do not use Incruse Ellipta to treat asthma)
- if you have heart problems
- if you have an eye problem called narrow-angle glaucoma
- if you have enlarged prostate, difficulty urinating, or a blockage in the bladder
- if you have severe liver problems.
Consult your doctorif you think any of the above conditions apply to you.
Immediate breathing difficulties
If you experience chest tightness, coughing, wheezing, or difficulty breathing immediately after using your Incruse Ellipta inhaler:
stop using this medicine and seek medical attention immediately, as you may have a serious condition called paradoxical bronchospasm.
Eye problems during treatment with Incruse Ellipta
If you experience eye pain or discomfort, blurred vision for a time, halos, or colored images associated with redness of the eyes during treatment with Incruse Ellipta:
stop using this medicine and seek medical help immediately, as these signs may be due to an acute attack of narrow-angle glaucoma.
Children and adolescents
Do not give this medicine to children or adolescents under 18 years.
Other medicines and Incruse Ellipta
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. If you are not sure what your medicine contains, consult your doctor or pharmacist.
In particular, tell your doctor or pharmacist if you are taking other long-acting medicines to treat similar respiratory problems to this medicine, such as tiotropium. Do not use Incruse Ellipta with these medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, consult your doctorbefore using this medicine. If you are pregnant, do not use this medicine unless your doctor tells you to.
It is not known if the components of Incruse Ellipta can be excreted in breast milk. If you are breastfeeding, consult your doctorbefore using Incruse Ellipta.
If you are breastfeeding, do not use this medicine unless your doctor tells you to.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use machines.
Incruse Ellipta contains lactose
If your doctor has told you that you have an intolerance to some sugars, consult them before using this medicine.
3. How to use Incruse Ellipta
Follow the instructions for administration of this medicine exactly as your doctor has told you. If you are not sure, consult your doctor or pharmacist again.
The recommended doseis one inhalation every day, at the same time each day. You only need one inhalation per day, as the effect of this medicine lasts for 24 hours.
Do not use more doses than your doctor has told you to.
Use Incruse Elliptaregularly
It is very important that you use Incruse Ellipta every day, as your doctor has told you. This will help you not to have symptoms throughout the day and night.
Do notuse this medicine to relieve a sudden attack of shortness of breath or wheezing. If you have this type of attack, you should use a fast-acting "rescue" inhaler (such as salbutamol).
How to use the inhaler
To get the full information, read the "Step-by-step instructions for use" at the end of this package leaflet.
The administration of Incruse Ellipta is by inhalation. To use Incruse Ellipta, breathe it into your lungs through your mouth using the Ellipta inhaler.
If your symptoms do not improve
If your COPD symptoms (shortness of breath, wheezing, coughing) do not improve or worsen, or if you are using your fast-acting "rescue" inhaler more often than usual:
contact your doctor as soon as possible.
If you use more Incruse Ellipta than you should
If you accidentally use too much medicine, contact your doctor or pharmacist immediately, as you may need medical attention. If possible, show them the inhaler, packaging, or package leaflet. You may notice that your heart beats faster than normal, you have vision changes, or your mouth is dry.
If you forget to use Incruse Ellipta
Do not inhale a double dose to make up for forgotten doses.Inhale the next dose at the usual time.
If you have wheezing or shortness of breath, use your fast-acting "rescue" inhaler (such as salbutamol) and seek medical advice.
If you stop using Incruse Ellipta
Use this medicine for as long as your doctor recommends. It will only be effective for as long as you use it. Do not stop using it until your doctor tells you to, even if you feel better, as your symptoms may worsen.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions
If after using Incruse Ellipta you have any of the following symptoms, stop using thismedicine and tell your doctor immediately:
Uncommon(may affect up to 1 in 100people):
- itching
- rash (hives) or redness.
Rare(may affect up to 1 in 1,000people):
- wheezing (a whistling sound when breathing), coughing, or difficulty breathing
- a sudden feeling of weakness or dizziness (which may cause fainting or loss of consciousness)
Other side effects:
Common(may affect up to 1 in 10people):
- fast heart rate
- pain when urinating and increased frequency (may be signs of a urinary tract infection)
- common cold
- nose and throat infection
- coughing
- feeling of pressure or pain in the cheeks and forehead (may be symptoms of sinusitis, an inflammation of the nasal passages)
- headache
- constipation
- mouth and throat pain.
Uncommon(may affect up to 1 in 100people):
- irregular heartbeat
- sore throat
- dry mouth
- change in taste
- hoarseness.
Rare(may affect up to 1 in 1,000people):
- eye pain.
Frequency not known(frequency cannot be estimated from the available data):
- decreased vision or eye pain due to increased eye pressure (possible signs of glaucoma)
- blurred vision
- increased eye pressure
- difficulty and pain when urinating, this may be a sign of bladder obstruction or urinary retention
- dizziness.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Incruse Ellipta
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging, tray, and inhaler, after EXP. The expiry date is the last day of the month stated.
Keep the inhaler in the sealed tray to protect it from moisture and only remove it immediately before the first use. Once the tray is opened, the inhaler can be used for a period of 6 weeks, counting from the date of opening of the tray. Write the date when the inhaler should be discarded on the space provided on the inhaler label. The date should be noted as soon as the inhaler is removed from the tray.
Do not store above 30 °C.
If you store it in the refrigerator, let the inhaler return to room temperature at least one hour before use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
Incruse Ellipta composition
The active ingredient is umeclidinium (as bromide).
Each inhalation provides a delivered dose (dose that comes out of the mouthpiece) of 55 micrograms of umeclidinium (equivalent to 65 micrograms of umeclidinium bromide).
The other ingredients are lactose monohydrate (see section “Incruse Ellipta contains lactose” in section 2) and magnesium stearate.
Appearance of the product and container contents
Incruse Ellipta is a powder for inhalation (single-dose).
The Ellipta inhaler consists of a gray plastic body, a light green mouthpiece cover, and a dose counter. It is packaged in a laminated aluminum tray with a foldable aluminum foil. The tray contains a bag with a desiccant to reduce moisture in the container.
The active ingredient is presented as a white powder in a blister inside the inhaler. Incruse Ellipta is available in packs of one inhaler containing 7 or 30 doses and in multipacks of 90 doses (3 inhalers of 30 doses). Only some pack sizes may be marketed.
Marketing authorization holder:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
Ireland
D24 YK11
Manufacturer:
Glaxo Wellcome Production
Zone Industrielle No.2
23 Rue Lavoisier
27000 Evreux
France
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v. Tél/Tel: + 32 (0) 10 85 52 00 | Lietuva UAB “BERLIN-CHEMIE MENARINI BALTIC” Tel: +370 52 691 947 |
| Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgique/Belgien Tél/Tel: + 32 (0) 10 85 52 00 |
Ceská republika GlaxoSmithKline, s.r.o. Tel: + 420 222 001 111 | Magyarország Berlin-Chemie/A. Menarini Kft. Tel.: +36 23501301 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Trading Services Limited Tel: +356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel.: + 49 (0)89 36044 8701 | Nederland GlaxoSmithKline BV Tel: + 31 (0) 33 2081100 |
Eesti OÜ Berlin-Chemie Menarini Eesti Tel: +372 667 5001 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλáδα Menarini Hellas A.E. Τηλ: +30 210 83161 11-13 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 |
France Laboratoire GlaxoSmithKline Tél: + 33 (0)1 39 17 84 44 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Hrvatska Berlin-Chemie Menarini Hrvatska d.o.o. Tel: +385 1 4821 361 | România GlaxoSmithKline Trading Services Limited Tel: +40 800672524 |
Ireland GlaxoSmithKline Trading Services Limited Tel: + 353 (0)1 4955000 | Slovenija Berlin-Chemie / A. Menarini Distribution Ljubljana d.o.o. Tel: +386 (0)1 300 2160 |
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika Berlin-Chemie / A. Menarini Distribution Slovakia s.r.o. Tel: +421 2 544 30 730 |
Italia GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741 111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 (0)10 30 30 30 |
Κúπρος GlaxoSmithKline Trading Services Limited Τηλ: +357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija SIA Berlin-Chemie/Menarini Baltic Tel: +371 67103210 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
Step-by-step instructions for use
What is the Ellipta inhaler?
The first time you use Incruse Ellipta, you do not need to check that the inhaler is working correctly, as it contains pre-measured doses and is ready to use directly.
Your Incruse Ellipta inhaler pack contains:
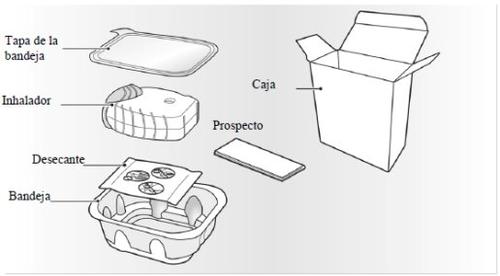
The inhaler is packaged in a tray. Do not open the tray until you are ready to start using your new inhaler. When you are ready to use the inhaler, remove the lid to open the tray. The tray contains a bag with a desiccantto reduce moisture. Discard the desiccant bag, do notopen it, ingest it, or inhale it.
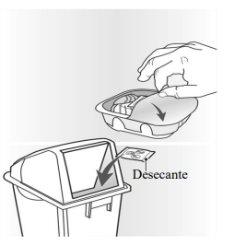
When you remove the inhaler from its tray, it will be in the “closed” position. Do not open the inhaler until you are ready to inhale a dose of medicine.When the tray is opened, the “Discard by” date should be noted on the space provided on the inhaler label. The “Discard by” date is 6 weeks from the date of opening the tray. After this date, the inhaler should not be used. The tray can be discarded once it is opened.
If stored in the refrigerator, let the inhaler reach room temperature for at least one hour before use.
The step-by-step instructions for using the Ellipta inhaler provided below can be used for both the 30-dose inhaler (30 days of treatment) and the 7-dose inhaler (7 days of treatment).
- Read this information before starting
If the inhaler cap is opened and closed without inhaling the medicine, the dose will be lost.The lost dose will be retained safely inside the inhaler, but it will not be available for inhalation.
It is not possible to accidentally administer an additional dose or a double dose through an inhalation.
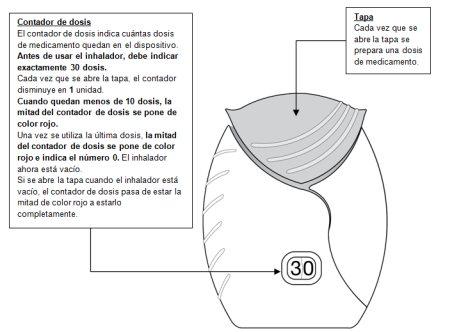
- Prepare a dose
Wait to open the inhaler cap until you are ready to inhale a dose.
Do not shake the inhaler.
- Slide the cap down until you hear a ‘click’.
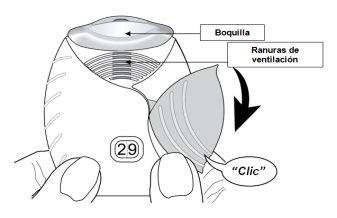
Now, the medicine is ready to be inhaled.
As confirmation, the dose counter decreases by 1unit.
- If the dose counter does not decrease when you hear the ‘click’, the inhaler will not release the dose of medicine.
Take it to the pharmacist and ask for help.
- Inhale your medicine
- While holding the inhaler away from your mouth, breathe out as much as you can.
Do notbreathe out into the inhaler.
- Place the mouthpiece between your lips, and close them firmly around the mouthpiece.
Do notblock the ventilation slots with your fingers.
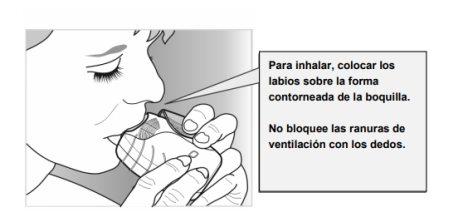
- Take a long, steady, and deep breath in. Hold your breath for as long as you can (at least 3-4 seconds).
- Remove the inhaler from your mouth.
- Breathe out slowly and gently.
You may not be able to taste or feel the medicine, even when using the inhaler correctly.
Beforeclosing the cap, the inhaler mouthpiece can be cleaned using a dry cloth.
- Close the inhaler
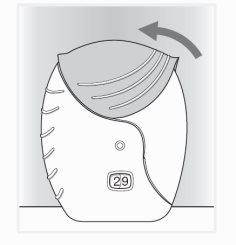
- Slide the cap up, to the top, to cover the mouthpiece.
- Country of registration
- Average pharmacy price45.27 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INCRUSE ELLIPTA 55 mcg INHALATION POWDER, SINGLE-DOSEDosage form: PULMONARY INHALATION, 55 microgramsActive substance: umeclidinium bromideManufacturer: Glaxosmithkline Trading Services LimitedPrescription requiredDosage form: PULMONARY INHALATION, 0.0040 g/aerosolActive substance: ipratropium bromideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 20 µgActive substance: ipratropium bromideManufacturer: Boehringer Ingelheim Espana S.A.Prescription required
Online doctors for INCRUSE ELLIPTA 55 mcg INHALATION POWDER, SINGLE-DOSE
Discuss questions about INCRUSE ELLIPTA 55 mcg INHALATION POWDER, SINGLE-DOSE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















