INCRELEX 10 mg/ml INJECTABLE SOLUTION

How to use INCRELEX 10 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
INCRELEX 10 mg/ml Solution for Injection
Mecasermin
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is INCRELEX and what is it used for
- What you need to know before you use INCRELEX
- How to use INCRELEX
- Possible side effects
- Storing INCRELEX
- Contents of the pack and other information
1. What is INCRELEX and what is it used for
- INCRELEX is a liquid solution that contains mecasermin, which is a synthetic insulin-like growth factor-1 (IGF-1), similar to the IGF-1 produced by your body.
- It is used to treat children and adolescents from 2 to 18 years who are too short for their age because their body cannot produce enough IGF-1. This condition is called primary IGF-1 deficiency.
2. What you need to know before you use INCRELEX
Do not use INCRELEX
- If you currently have any tumor or abnormal growth, whether cancerous or non-cancerous
- If you have had cancer in the past
- If you have any condition that may increase the risk of developing cancer
- If you are allergic (hypersensitive) to mecasermin or any of the other ingredients of this medicine (listed in section 6).
- In neonates or premature infants because it contains benzyl alcohol.
Warnings and precautions
There is an increased risk of developing tumors (both cancerous and non-cancerous) in children and adolescents treated with INCRELEX. Since mecasermin may play a role in the development of cancer, inform your doctor immediately if any new tumor, skin lesion, or unexpected symptom appears during or after treatment.
Consult your doctor or pharmacist before starting treatment with INCRELEX
- If you have a lateral deviation of the spine (scoliosis). It is advisable to monitor the progression of scoliosis.
- If you develop a limp or pain in the knee or hip
- If you have an increase in the size of the tonsils (tonsillar hypertrophy). It is advisable to undergo periodic check-ups.
- If you have symptoms that indicate an increase in pressure in the brain (intracranial hypertension), such as visual changes, headache, nausea, and/or vomiting; consult your doctor.
- If you experience a localized reaction at the injection site or a generalized allergic reaction to INCRELEX. Go to the doctor as soon as possible if you develop a localized skin rash. Seek immediate medical attention if you have a generalized allergic reaction (hives, breathing problems, fainting or collapse, and a general feeling of discomfort).
- If you have stopped growing (bone growth plates have closed). In this case, INCRELEX cannot help you grow and you should not use it.
Children under 2 years
The use of this medicine has not been studied in children under 2 years, therefore it is not recommended.
Other medicines and INCRELEX
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Tell your doctor especially if you are being treated with insulin or other anti-diabetic medicines.
It may be necessary to adjust the dose of these medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
For all women of childbearing age, a negative pregnancy test is recommended before starting treatment with mecasermin. It is also recommended that all women of childbearing age use adequate contraceptive measures during treatment.
Treatment with mecasermin should be discontinued if pregnancy occurs.
Mecasermin should not be administered to a breastfeeding mother.
Driving and using machines
Mecasermin may cause hypoglycemia (a very common side effect, see section 4) that may affect your ability to drive and use machines, as your ability to concentrate or react may be reduced.
You should avoid any high-risk activity (e.g., driving, etc.) in the 2-3 hours following administration of the dose, especially at the beginning of treatment with INCRELEX and until a dose of INCRELEX is found that does not produce side effects that make these activities a risk.
INCRELEX contains benzyl alcohol and sodium
INCRELEX contains benzyl alcohol as a preservative, which may cause toxic and allergic reactions in infants and children up to 3 years of age.
This medicine contains less than 1 mmol of sodium (23 mg) per vial, which is considered essentially "sodium-free".
3. How to use INCRELEX
Follow the instructions for administration of this medicine exactly as indicated by your doctor.
Consult your doctor or pharmacist if you have any doubts.
The normal dose is 0.04 to 0.12 mg per kg of patient weight administered twice a day.
See the "Instructions for use" at the end of this leaflet.
Inject INCRELEX just under your skin a little before or after a meal or snack, as it may have insulin-like hypoglycemic effects and thus lower blood sugar levels (see hypoglycemia in section 4). Do not inject the dose of INCRELEX if you cannot eat for any reason. Do not try to compensate for the missed dose by administering a double dose the next time. The next dose should be administered as usual, with a meal or snack.
Inject INCRELEX just under your skin at some point on the arm, thigh, stomach area (abdomen), or buttocks. Never inject into a vein or muscle. Change the injection site each time.
Do not use INCRELEX if it is not transparent and colorless.
Treatment with mecasermin is long-term treatment. Consult your doctor for more information.
If you use more INCRELEX than you should
Mecasermin, like insulin, may lower blood sugar levels (see hypoglycemia in section 4).
If you have injected more INCRELEX than recommended, contact your doctor immediately.
An acute overdose may cause hypoglycemia (low blood glucose concentration).
Treatment of acute overdose of mecasermin should focus on reversing hypoglycemia. Food or sugary drinks should be ingested. If the patient is not conscious enough to take sugary drinks, an intramuscular injection of glucagon may be necessary to reverse hypoglycemia. Your doctor or nurse will teach you how to inject glucagon.
Prolonged overdose may cause an increase in size of certain parts of the body (e.g., hands, feet, parts of the face) or excessive growth of the entire body. If you suspect prolonged overdose, contact your doctor immediately.
If you forget to use INCRELEX
Do not take a double dose to make up for forgotten doses.
If you have missed a dose, the next dose should not be larger to make up for it. The next dose should be administered as usual, with a meal or snack.
If you stop treatment with INCRELEX
Stopping or prematurely ending treatment with mecasermin may reduce the success of growth therapy. Ask your doctor before stopping treatment.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet.
The most frequently reported side effects with mecasermin are: low blood sugar (hypoglycemia), vomiting, reactions at the injection site, headache, and middle ear infections. Serious allergic reactions have also been reported with INCRELEX. If you experience any of these effects, follow the advice given for each effect in the following sections.
Frequency not known (frequency cannot be estimated from available data)Cancerous and non-cancerous tumors
In patients treated with INCRELEX, an increased incidence of cancerous and non-cancerous tumors has been reported. The risk of developing such tumors may be higher if INCRELEX is used for a condition other than the one indicated in section 1 or at a dose higher than recommended in section 3.
Severe allergic reactions (anaphylaxis)
After using mecasermin, generalized urticaria, difficulty breathing, drowsiness, facial and/or throat swelling have been reported. If you develop a severe allergic reaction, you should stop INCRELEX immediately and seek urgent medical attention.
Local allergic reactions at the injection site (itching, urticaria) have also been reported.
Hair loss (alopecia)
With the use of mecasermin, hair loss has also been reported.
Very common (may affect more than 1 in 10 people)
Low blood sugar (hypoglycemia)
Mecasermin may lower blood sugar levels. Signs of low blood sugar are: drowsiness, fatigue, restlessness, hunger, irritability, concentration problems, sweating, nausea, and rapid or irregular heartbeat.
Severe hypoglycemia can cause unconsciousness, convulsions/seizures, or death. Stop INCRELEX immediately and seek urgent medical attention if you develop convulsions/seizures or unconsciousness.
If you take INCRELEX, you should avoid participating in high-risk activities (such as intense physical activity) in the 2-3 hours following injection of INCRELEX, especially at the start of treatment with INCRELEX.
Before starting treatment with INCRELEX, your doctor or nurse will explain how to treat hypoglycemia. You should always have a source of sugar, such as orange juice, glucose gel, sweets, or milk, on hand in case symptoms of hypoglycemia appear. In case of severe hypoglycemia, if you do not respond and cannot take a sugary drink, an injection of glucagon should be administered. Your doctor or nurse will teach you how to administer the injection. The injection of glucagon increases blood glucose levels. It is important that you follow a balanced diet that includes, in addition to sugary foods, proteins, and fats such as meat and cheese.
Hypertrophy at the injection site (the tissue at the injection site increases in size) and hematomas
This can be avoided by changing the injection site each time (rotation of the injection site).
Digestive system
With treatment with mecasermin, cases of vomiting and pain in the upper abdomen have occurred.
Infections
In children treated with mecasermin, middle ear infections have been observed.
Musculoskeletal system
With treatment with mecasermin, cases of joint pain and limb pain have occurred.
Nervous system
With treatment with mecasermin, cases of headache have occurred.
Frequent (may affect up to 1 in 10 people):
Seizures
With treatment with mecasermin, cases of seizures (fits) have been observed.
With treatment with mecasermin, cases of dizziness and tremors have also been reported.
Cardiac anomalies
With treatment with mecasermin, cases of rapid heartbeat and abnormal heart sounds have been reported.
High blood sugar (hyperglycemia)
With treatment with mecasermin, high blood sugar has also been observed.
Increased size of the tonsils/adenoids
Mecasermin may increase the size of the tonsils/adenoids. Signs of increased size of the tonsils/adenoids include: snoring, difficulty breathing or swallowing, sleep apnea (a disorder in which there are brief episodes of suspension of breathing during sleep), or presence of fluid in the middle ear, in addition to middle ear infections. Sleep apnea can cause excessive daytime sleepiness. Go to the doctor if these symptoms are bothersome. Your doctor should regularly examine your tonsils.
Increased size of the thymus
With treatment with mecasermin, an increase in the size of the thymus (a specialized organ of the immune system) has been observed.
Papilledema
During treatment with mecasermin, your doctor or an optician may observe inflammation of the back of the eye (due to increased pressure in the brain).
Hearing loss (hypacusis)
With treatment with mecasermin, hearing loss (hypacusis), ear pain, and fluid in the middle ear have been observed. Inform your doctor if you develop hearing problems.
Worsening of scoliosis (caused by rapid growth)
If you have scoliosis, it will be necessary to undergo frequent check-ups to see if the curvature of the spine has increased. With treatment with mecasermin, cases of muscle pain have also been observed.
Reproductive system
With treatment with mecasermin, cases of breast enlargement have been observed.
Digestive system
With treatment with mecasermin, cases of abdominal pain have occurred.
Changes in skin and hair
With treatment with mecasermin, cases of skin thickening, moles, and changes in hair texture have been observed.
Reactions at the injection site
Reactions reported with treatment with mecasermin include pain, irritation, bleeding, hematoma, redness, and hardening. Injection site reactions can be avoided by changing the injection site each time (rotation of the injection site).
Uncommon (may affect up to 1 in 100 people)
Increased intracranial pressure (intracranial hypertension)
INCRELEX may sometimes cause a temporary increase in intracranial pressure. Possible symptoms of intracranial hypertension include visual changes, headache, nausea, and/or vomiting. Inform your doctor immediately if you experience any of these symptoms. Your doctor will check if it is due to intracranial hypertension. If this is the case, your doctor may decide to stop or temporarily reduce treatment with mecasermin. Mecasermin can be administered again when the episode has passed.
Cardiac anomalies
In some patients treated with mecasermin, a heart examination (echocardiogram) has revealed an increase in the size of the heart muscle and abnormalities in the functioning of the heart valves. Your doctor may perform an echocardiogram before, during, and after treatment with mecasermin.
Reactions at the injection site
With treatment with INCRELEX, reactions have been reported that include skin rash, swelling, and fat lumps. Injection site reactions can be avoided by changing the injection site each time (rotation of the injection site).
Weight gain
With treatment with mecasermin, cases of weight gain have been reported.
Other uncommon side effects with treatment with mecasermin include depression, nervousness.
Reporting of side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing INCRELEX
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the vial perfectly closed to protect it from light.
After first use, the vial can be stored for up to 30 days between 2°C and 8°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of INCRELEX
- The active ingredient is mecasermin. One milliliter contains 10 mg of mecasermin. Each vial contains 40 mg of mecasermin.
- The other components are: benzyl alcohol, sodium chloride, polysorbate 20, glacial acetic acid, sodium acetate, and water for injectable preparations (see section 2 "INCRELEX contains benzyl alcohol and sodium").
Appearance of the Product and Container Contents
INCRELEX is a clear and colorless injectable solution (injection) supplied in a closed glass vial with a stopper and a cap. The vial contains 4 ml of solution.
Package size of 1 vial.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Ipsen Pharma
65, quai Georges Gorse
92100 Boulogne-Billancourt
France
Manufacturer:
Beaufour Ipsen Industrie
Rue d'Ethe Virton
28100 Dreux
France
You can request more information about this medication by contacting the local representative of the Marketing Authorization Holder:
Belgium/Luxembourg Ipsen NV Guldensporenpark 87 B-9820 Merelbeke Belgium Tel: + 32 9 243 96 00 | Italy Ipsen SpA Via del Bosco Rinnovato n.6 Milanofiori Nord Palazzo U7 20090 Assago (Mi) Tel: + 39 - 02 - 39 22 41 |
Romania Ipsen Pharma Str. Grigore Alexandrescu nr 59, cladirea HQ Sector 1, 010623, Bucuresti, Romania Tel/Phone: + 40 (021) 231 27 20 | Czech Republic Ipsen Pharma, s.r.o. Olbrachtova 2006/9 140 00 Praha 4 Tel: + 420 242 481 821 |
Denmark, Norway, Finland, Sweden, Iceland Institut Produits Synthèse (IPSEN) AB Kista Science Tower Färögatan 33 SE - 164 51 Kista Sweden Tel/Phone: +46 8 451 60 00 | Germany, Austria Ipsen Pharma GmbH Einsteinstrasse 174 D-81677 München Tel.: +49 89 262043289 |
Estonia Centralpharma Communications OÜ Selise 26-11, 13522, Tallinn Tel: +372 6015540 | Greece, Cyprus, Malta Ipsen Μονοπρ?σωπη EΠΕ Αγ. Δημητρ?ου 63 ?λιμος GR-17456 Αθ?να Greece Tel: + 30 - 210 - 984 3324 |
Spain Ipsen Pharma, S.A. Torre Realia, Plaza de Europa, 41 - 43 08908 L’Hospitalet de Llobregat Barcelona Tel: + 34 - 936 - 858 100 | France Ipsen Pharma 65 quai Georges Gorse F-92100 Boulogne-Billancourt Tel: + 33 - 1 - 58 33 50 00 |
Latvia Ipsen Pharma parstavnieciba Latvija Kalnciema iela 33-5 Riga LV 1046 Tel: +371 67622233 | Lithuania, Croatia Ipsen Pharma Lietuvos filialas ojo Forto 47 LT-48100 Kaunas Tel. + 370 37 337854 |
Hungary Ipsen Pharma SAS Magyarországi Kereskedelmi Képviselet Árbóc utca 6. H-1133 Budapest Tel: +36 1 555 5930 | Netherlands Ipsen Farmaceutica B.V. Taurusavenue 33 B NL-2132 LS Hoofddorp Tel: + 31 23 55 41 600 |
Poland Ipsen Poland Sp. z o.o. Al. Jana Pawla II 29 PL-00-867 Warszawa Tel.: + 48 (0) 22 653 68 00 | Portugal Ipsen Portugal - Produtos Farmacêuticos S.A. Alameda Fernão Lopes, no 16A-1oB P-1495 - 190 Algés Tel: + 351 - 21 - 412 3550 |
Slovenia PharmaSwiss d.o.o Brodišce 32 SI-1236 Trzin Tel: + 386 1 236 47 00 | United Kingdom Ipsen Ltd. 190 Bath Road Slough, Berkshire SL1 3XE Tel: + 44 - (0)1753 - 62 77 00 |
Ireland Ipsen Pharmaceuticals Ltd. Blanchardstown Industrial Park Blanchardstown IRL-Dublin 4 Tel: + 353 - 1 - 8098200 | Slovak Republic Liek s.r.o. Hviezdoslavova 19 SK-903 01 Senec Tel: + 421 245 646 322 |
Date of Last Revision of this Prospectus:
This medicinal product has been authorized under "exceptional circumstances".
This type of authorization means that due to the rarity of the disease, it has not been possible to obtain complete information on this medicinal product.
The European Medicines Agency will review any new information on the medicinal product that may become available annually, and this prospectus will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
This prospectus can be found on the European Medicines Agency website in all languages of the European Union/European Economic Area.
<---------------------------------------------------------------------------------------------------------------------->
INSTRUCTIONS FOR USE
INCRELEX should be administered using sterile disposable needles and syringes that your doctor/pharmacist or nurse can provide. Use small volume syringes that allow you to withdraw the prescribed dose from the vial with reasonable accuracy.
Preparation of the Dose
- Wash your hands before preparing INCRELEX for injection.
- Use a new disposable needle and syringe each time you administer a dose. Do not use the syringes and needles more than once. Dispose of them properly in a puncture-proof container (e.g., a biological hazardous waste container), a rigid plastic container (e.g., a detergent bottle), or a metal container (e.g., an empty coffee can). Never share needles and syringes.
- Check that the liquid is clear and colorless. Do not use it after the expiration date (which appears on the label after EXP and refers to the last day of the month), or if it is cloudy or you notice that it contains particles. If a vial is frozen, dispose of it properly. Ask your pharmacist how to dispose of unused medications.
- If you are using a new vial, remove the protective cap. Do not remove the rubber stopper.
- Rub the rubber stopper of the vial with a cotton swab soaked in alcohol to prevent the vial from becoming contaminated with germs that may be introduced when the needle is inserted repeatedly (see Figure 1).
|
Figure 1: Rub the top of the stopper with alcohol |
- Before inserting the needle into the vial, pull the plunger back to draw a volume of air into the syringe equal to the prescribed dose. Insert the needle through the rubber stopper of the vial and push the plunger to inject air into the vial (see Figure 2).
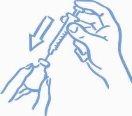
Figure 2: Inject air into the vial
- Leave the syringe in the vial and turn both upside down. Hold the syringe and vial firmly (see Figure 3).
Figure 3: Prepare for withdrawal |
- Make sure the tip of the needle is in the liquid (see Figure 4). Pull the plunger to draw the correct dose into the syringe (see Figure 5).

Figure 4: Tip in the liquid | Figure 5: Withdraw the correct dose |
- Before removing the needle from the vial, check for air bubbles in the syringe. If there are air bubbles, hold the vial and syringe with the needle straight up and gently tap the side of the syringe until the bubbles float to the top. Expel the bubbles with the plunger and withdraw the liquid again until you reach the correct dose (see Figure 6).

Figure 6: Expel air bubbles and refill the syringe
- Remove the needle from the vial and replace the protective cap. Do not let the needle touch anything. You are now ready to inject (see Figure 7).

Figure 7: Ready to inject
Injection of the Dose:
Inject INCRELEX following the doctor's instructions.
Do not administer the injection if you will not be able to eat a little before or after the injection.
- Choose an injection site, either the arm, thigh, buttock, or abdomen (see below). It is a good idea to change the injection site each time (rotate the injection site).
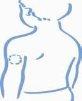
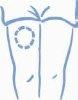

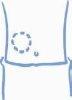
Arm Thigh Buttock Abdomen
- Use alcohol or soap and water to clean the skin area where you will inject. The injection site should be dry before injection.
- Gently pinch the skin. Insert the needle as your doctor taught you. Release the skin (see Figure A).
Figure A: Pinch the skin gently and inject as explained |
- Slowly push the syringe plunger all the way in, trying to inject all the liquid. Pull the needle straight out and press the injection site gently with a swab or cotton ball for a few seconds. Do not rub the area(see Figure B).

Figure B: Press (do not rub) with a swab or cotton ball
- Follow your doctor's instructions for disposing of the needle and syringe. Do not put the cap back on the syringe. The used needle and syringe should be placed in a puncture-proof container (e.g., a biological hazardous waste container), a rigid plastic container (e.g., a detergent bottle), or a metal container (e.g., an empty coffee can). These containers should be sealed and disposed of properly as your doctor explains.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INCRELEX 10 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 5.3 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 0.2 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Online doctors for INCRELEX 10 mg/ml INJECTABLE SOLUTION
Discuss questions about INCRELEX 10 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions