
IMMUNINE 1200 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR FOR INFUSION

How to use IMMUNINE 1200 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
IMMUNINE 1200 IU
Powder and solvent for solution for injection or infusion
human blood coagulation factor IX
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is IMMUNINE and what is it used for
- What you need to know before you use IMMUNINE
- How to use IMMUNINE
- Possible side effects
- Storage of IMMUNINE
- Contents of the pack and other information
1. What is IMMUNINE and what is it used for
IMMUNINE is a concentrate of coagulation factor IX. It replaces the factor IX that is missing or not working properly in hemophilia B. Hemophilia B is a hereditary and sex-linked bleeding disorder caused by a deficiency of factor IX. It causes severe bleeding into joints, muscles, and internal organs, either spontaneously or as a result of accidental or surgical trauma. Administration of IMMUNINE temporarily corrects the factor IX deficiency and reduces the tendency to bleed.
IMMUNINE is used for the treatment and prophylaxis of bleeding episodes in patients with congenital hemophilia B.
IMMUNINE is indicated for all age groups, from children over 6 years to adults.
There are not enough data to recommend the use of IMMUNINE in children under 6 years of age.
2. What you need to know before you use IMMUNINE
Do not use IMMUNINE
- If you are allergicto human coagulation factor IX or to any of the other components of this medicine (listed in section 6).
- If you have a known allergy to heparin or have had an abnormal decrease in the number of blood cells involved in the formation of blood clots caused by heparin administration (heparin-induced thrombocytopenia).
After adequate treatment of these situations, IMMUNINE should only be used in case of life-threatening bleeding episodes.
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with IMMUNINE.
When allergic reactions occur:
There is a rare possibility that you may experience a sudden and severe allergic reaction (anaphylactic reaction) to IMMUNINE.
If you detect one or more of the following symptoms, stop administration immediately and seek medical assistance at once. They may be signs of anaphylactic shock and require immediate urgent treatment.
- redness of the skin
- rash
- hives
- itching all over the body
- swelling of lips and tongue
- difficulty breathing
- difficulty inhaling or exhaling due to constriction of the airways
- chest tightness
- general discomfort
- dizziness
- decrease in blood pressure
- loss of consciousness
When monitoring is required:
- It is possible that your doctor may want to perform some tests to ensure that the dose you receive is sufficient to achieve and maintain adequate factor IX levels in the blood.
- Your doctor will monitor you closely to detect possible complications:
- if you receive high doses of IMMUNINE
- if you are prone to developing thrombosis. In this case, you will receive lower levels of factor IX, the active ingredient of IMMUNINE.
When bleeding continues:
- If your bleeding does not come under control with IMMUNINE, consult your doctor immediately. It is possible that you have developed factor IX inhibitors. Factor IX inhibitors are antibodies present in the blood that counteract the effect of factor IX. This reduces the effectiveness of IMMUNINE in treating bleeding episodes. Your doctor will perform the necessary tests to confirm this.
- There may be a connection between the development of factor IX inhibitors and allergic reactions. Patients with factor IX inhibitors may have a higher risk of severe allergic reactions (anaphylaxis). Therefore, in patients who suffer an allergic reaction, the presence of a factor IX inhibitor should be investigated.
Tell your doctor if you have heart or liver disease or if you have recently undergone major surgery, as there is a higher risk of blood clotting complications.
Safety information regarding infectious agent transmission
When human plasma or blood-derived medicines are administered, certain measures must be taken to prevent infections from being transmitted to patients. These measures include:
- careful selection of donors to exclude those who are at risk of being carriers of infectious diseases
- testing for specific infection markers in individual donations and plasma pools to detect possible viruses or infections
- inclusion of stages in the manufacturing process to eliminate/inactivate viruses.
Despite this, when human blood or plasma-derived medicines are administered, the possibility of transmitting infectious agents cannot be completely excluded. This also applies to emerging or unknown viruses or other types of infections.
These measures are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and for the non-enveloped hepatitis A virus.
The measures taken may have limited value against other non-enveloped viruses such as parvovirus B19 (the virus that causes erythema infectiosum).
Parvovirus B19 infection can be severe for a pregnant woman (fetal infection) and for individuals whose immune system is depressed or for patients with certain types of anemia (e.g., sickle cell anemia or hemolytic anemia).
Your doctor may recommend that you be vaccinated against hepatitis A and hepatitis B if you are regularly or repeatedly administered human plasma-derived medicines.
It is strongly recommended that, each time a dose of IMMUNINE is administered, a record be kept of the name of the medicine and the batch number administered in order to maintain a record of the batches used.
Children
There are not enough data to recommend the use of IMMUNINE in children under 6 years of age.
Using IMMUNINE with other medicinesTell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
No interactions of IMMUNINE with other medicines are known.
Pregnancy and breastfeeding
Hemophilia B is rare in women. Therefore, there is currently no experience with the use of IMMUNINE during pregnancy and breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Your doctor will decide whether you can use Immunine during pregnancy and breastfeeding.
Driving and using machines
No effects on the ability to drive and use machines have been observed.
IMMUNINE contains sodium chloride and sodium citrate
This medicine contains 41 mg of sodium (the main component of cooking/table salt) in each vial. This is equivalent to 2% of the maximum recommended daily intake of sodium for an adult.
3. How to use IMMUNINE
Treatment should be initiated and supervised by a doctor experienced in the treatment of hemophilia B.
Your doctor will decide what dose is appropriate for you. He will calculate the dose according to your individual needs. Tell your doctor if you think the effect of Immunine is too strong or too weak.
Use in children
There are not enough data to recommend the use of IMMUNINE in children under 6 years of age.
Monitoring by your doctor
Your doctor will perform the appropriate laboratory tests at regular intervals to ensure that you have sufficient factor IX levels. This is especially important if you are going to undergo major surgery or life-threatening bleeding.
Patients with inhibitors
If the factor IX level in the blood does not reach the expected levels, or if bleeding is not controlled after an adequate dose, the presence of factor IX inhibitors should be suspected. Your doctor will check for the presence of inhibitors by performing the appropriate tests. In case of inhibitor development, contact a specialized hemophilia center.
If you have developed factor IX inhibitors, you may need a higher dose of IMMUNINE to control bleeding. If this dose does not control your bleeding, your doctor may consider using an alternative medicine. Do not increase the total dose of IMMUNINE you use to control your bleeding without consulting your doctor.
Frequency of administration
Your doctor will tell you how often and at what intervals IMMUNINE should be administered. He will calculate it for your individual case, depending on your response to IMMUNINE.
Route and/or method of administration
IMMUNINE is administered slowlyinto a vein (intravenously) after preparing the solution with the provided solvent.
Before administration, IMMUNINE should not be mixed with other medicines. This may affect the efficacy and safety of the product.
Please follow your doctor's instructions strictly.
The rate of administration will depend on the discomfort you may feel, without exceeding 2 ml per minute.
- Use only the administration equipment provided. If other administration equipment is used, incorrect dosing may occur as a result of IMMUNINE adsorption to the inner surfaces of some infusion equipment.
- If you are also receiving other products through the same venous access, you mustflush the access with a suitable solution, such as physiological saline, before and afteradministration of IMMUNINE.
- IMMUNINE must be reconstituted only immediately before administration, and the solution must be used immediately. (The solution does not contain preservatives). The infusion must be completed within 3 hours of reconstitution.
- The injectable solution is clear or slightly milky (opalescent). Do not use cloudy solutions or those containing visible particles.
- Any unused solution must be disposed of properly.
Reconstitution of the powder to prepare an injectable solution:
Prepare the solution in the cleanest and most sterile conditions possible!
- Warm the closed vial with the rubber stopper containing the solvent (sterilized water for injectable preparations) to room temperature (max. 37°C).
- Remove the protectors from the vials with rubber stoppers containing the powder and solvent (fig. A) and clean the rubber stoppers of both vials.
- Remove the seal covering one end of the transfer needle by twisting and pulling it. Insert the needle through the rubber stopper of the solvent vial (fig. B and C).
- Remove the seal covering the other end of the transfer needle, taking care not to touch the exposed end.
- Invert the solvent vial over the powder vial and insert the free end of the transfer needle into the powder vial, piercing the stopper (fig. D). The vacuum in the powder vial will aspirate the solvent.
- After all the solvent has passed into the powder vial, separate the two vials by removing the transfer needle from the powder vial (fig. E). Gently swirl the powder vial to accelerate dissolution.
- Once the powder is completely dissolved, insert the provided venting needle (Fig. F) and any foam that may have formed will disappear. Remove the venting needle.
Injection/Infusion:
Prepare the solution in the cleanest and most sterile conditions possible!
- Remove the protector from the provided filter needle by twisting and pulling it, and attach it to a sterile disposable syringe. Aspirate the solution with the syringe (fig. G).
- Separate the syringe from the filter needle and inject the solution slowly (maximum rate of 2 ml per minute) intravenously with the provided infusion equipment (or with the included disposable needle).
If the product is administered by infusion, use the disposable winged infusion set with an appropriate filter.
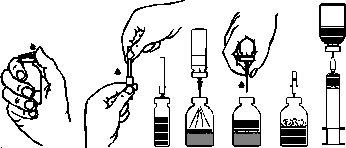
fig.A fig.B fig.C fig.D fig.E fig.F fig.G
Duration of treatment
Treatment with IMMUNINE should normally be continued for life.
If you use more IMMUNINE than you should:
Tell your doctor. Symptoms of overdose with factor IX have not been reported.
If you forget to use IMMUNINE
- Do not take a double dose to make up for forgotten doses.
- Continue with the next administration immediately and follow the regular intervals as indicated by your doctor.
If you stop treatment with IMMUNINE
Do not decide to stop treatment with IMMUNINE without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You must seek medical help immediately if you experience any of the following serious side effects
- severe allergic reaction (anaphylactic reaction). Stop administration immediately and seek medical assistance at once if you experience any of the following symptoms. Pay special attention if your doctor detects inhibitors in your blood.
- redness of the skin
- rash
- hives
- itching all over the body
- swelling of lips and tongue
- difficulty breathing
- difficulty inhaling or exhaling due to constriction of the airways
- chest tightness
- general discomfort
- dizziness
- decrease in blood pressure
- sudden swelling of the skin or mucous membranes, with or without difficulty swallowing or breathing (angioedema)
- formation of blood clots in small blood vessels throughout the body (disseminated intravascular coagulation (DIC))
- heart attack (myocardial infarction)
- rapid heartbeat (tachycardia)
- fall in blood pressure (hypotension)
- blood clots (thromboembolic events)
- blockage of a blood vessel by a blood clot (e.g., pulmonary embolism, venous thrombosis, arterial thrombosis, cerebral artery thrombosis)
- redness
- difficulty inhaling or exhaling due to constriction of the airways
- difficulty breathing
- a type of kidney disorder with symptoms such as swelling of the lips, face, and lower legs, with weight gain and protein elimination in the urine (nephrotic syndrome)
If your doctor detects inhibitors in your blood, you may be at specific risk of developing a disease called serum sickness. Stop administration immediately and seek medical assistance at once if you experience any of the following symptoms
- rash
- itching
- joint pain (arthralgia), especially in the fingers and toes
- fever
- swelling of the lymph nodes (lymphadenopathy)
- fall in blood pressure (hypotension)
- enlargement of the spleen (splenomegalia)
Other side effects
Uncommon side effects (may affect up to 1 in 100 people)
- throat irritation, sore throat, and cough (dry)
- skin rash and itching (pruritus)
- fever (pyrexia)
Side effects with frequency not known (cannot be estimated from the available data)
- headache
- restlessness
- tingling
- feeling of dizziness (nausea)
- vomiting
- hives all over the body (urticaria)
- chills
- hypersensitivity reactions
- burning and stinging sensation at the injection site
- lethargy
- redness
- chest tightness
The following side effects have been observed with other medicines in the same group:reduced or abnormal sensitivity (paresthesia).
Reporting of side effects
If you experience any side effects, talk to your doctor, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of IMMUNINE
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton. The expiry date refers to the last day of the month.
Store in a refrigerator (2°C-8°C). Do not freeze.
Keep the vial in the outer packaging to protect it from light.
During its validity period, IMMUNINE can be stored at room temperature up to 25°C for a maximum of 3 months only. Note on the product packaging the start and end of the storage period at room temperature (up to 25°C). IMMUNINE must be used within these three months. After the end of this period, IMMUNINE should not be refrigerated, but used immediately or discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package contents and additional information
Composition of IMMUNINE
Powder
- The active substance is human blood coagulation factor IX. 1 vial of powder for solution for injection contains 1200 IU of human blood coagulation factor IX. 1 ml of solution contains approximately 120 IU of human blood coagulation factor IX, when reconstituted with 10 ml of sterile water for injections.
- The other ingredients are sodium chloride and sodium citrate.
Solvent
- Sterile water for injections
Appearance and package contents of the product
Immunine is a white or pale yellow powder for preparing an injectable solution. After reconstitution with the provided solvent (sterile water for injections), a clear or slightly milky (opalescent) solution is obtained. If particles or coloration are observed or the solution is turbid, please do not use the product and contact Shire's Customer Service department.
Package size:1 x 1200 IU
Each carton contains:- 1 vial of IMMUNINE 1200 IU
- 1 vial with 10 ml of sterile water for injections
-1 transfer needle
-1 venting needle
-1 filter needle
-1 disposable needle
-1 disposable syringe (10 ml)
- 1 infusion set
Marketing authorisation holder and manufacturer:
Marketing authorisation holder:
Baxalta Innovations GmbH
Industriestrasse 67
1221 Vienna
Austria
Manufacturer:
Takeda Manufacturing Austria AG
Industriestrasse, 67
A-1221 Vienna, Austria
Local representative:
Takeda Farmacéutica España S.A.
Calle Albacete, 5, 9th floor
Edificio Los Cubos
28027 Madrid
Spain
Tel: +34 91 790 42 22
Marketing authorisation number: 69603
This medicinal product is authorised in the Member States of the European Economic Area under the following names:
Austria, Bulgaria, Czech Republic, Estonia, Germany, Latvia, Lithuania, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden: Immunine
Italy: Fixnove
Date of last revision of this leaflet:August 2022.
Detailed and up-to-date information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
---------------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only:
Posology and method of administration
Treatment should be initiated under the supervision of a doctor experienced in the treatment of haemophilia.
Posology
The dose and duration of replacement therapy depend on the severity of the factor IX deficiency, the location and extent of the bleeding, and the patient's clinical condition.
The number of units of factor IX administered is expressed in International Units (IU), which are related to the current WHO standard for factor IX products. The activity of factor IX in plasma is expressed either as a percentage (relative to normal human plasma) or in International Units (relative to an international standard of factor IX concentrates in plasma).
One International Unit (IU) of factor IX activity is equivalent to the amount of factor IX present in one ml of normal human plasma.
On-demand treatment
The calculation of the required dose of factor IX is based on the empirical finding that 1 International Unit (IU) of factor IX per kg of body weight increases the activity of factor IX in plasma by 1.1% of normal activity in patients 12 years and older.
The required dose is determined using the following formula:
Required IU = body weight (kg) x desired increase in factor IX (%)(IU/dl) x 0.9
The amount to be administered and the frequency of administration should always be guided by the individual clinical efficacy. Factor IX products are rarely needed to be administered more than once a day.
In the case of the following bleeding episodes, the activity of factor IX should not fall below the given plasma activity level (in % of normal or in IU/dl) during the corresponding time period.
In surgery and bleeding episodes, the following table can be used as a dosing guide:
Severity of bleeding/Surgical procedure | Required factor IX level (% of normal)(IU/dl) | Dosing frequency (hours)/Duration of treatment (days) |
Bleeding | ||
Early haemarthrosis or muscle or oral bleeding | 20-40 | Repeat every 24 hours for at least 1 day, until the bleeding episode, as indicated by pain, is resolved or healing is achieved. |
More extensive haemarthrosis, muscle bleeding or haematoma | 30–60 | Repeat infusion every 24 hours for 3-4 days or more, until pain ceases and acute disability is resolved. |
Life-threatening bleeding | 60-100 | Repeat infusion every 8-24 hours until the danger has passed. |
Surgery | ||
Minor surgery, including dental extractions | 30-60 | Every 24 hours, for at least 1 day, until healing. |
Major surgery | 80-100 (pre- and post-operative) | Repeat infusion every 8-24 hours until adequate wound healing and continue therapy for at least another 7 days to maintain a factor IX activity of 30% to 60%. |
Prophylaxis
For long-term prophylaxis against bleeding in patients with severe haemophilia B, the usual doses are 20 to 40 IU of factor IX per kg of body weight at intervals of 3 to 4 days.
In some cases, especially in younger patients, shorter dosing intervals or higher doses may be required.
During the course of treatment, it is recommended to determine the factor IX levels appropriately as a guide to the dose to be administered and the frequency of repeated infusions. In particular, in the case of major surgical interventions, exact monitoring of replacement therapy by means of coagulation analysis (plasma factor IX activity) is essential. The response to factor IX may vary in each individual patient, achieving different recovery levels in vivo and showing different half-lives.
Paediatric population
According to available clinical data, the recommended posology in the paediatric population can be performed in patients from 12 to 18 years of age. There are insufficient clinical data to provide a recommended posology in patients between 6 and 12 years of age.
Adverse reactions
Special populations
The use of IMMUNINE was investigated in paediatric patients with haemophilia B. The safety profile was similar to that of adults using IMMUNINE.
The use of IMMUNINE was investigated in two observational studies in children up to 6 years of age and in patients from 0 to 64 years of age with haemophilia B, respectively. The safety profile in children up to 6 years of age was similar to that of children over 6 years of age and to that of adults using IMMUNINE.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IMMUNINE 1200 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR FOR INFUSIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 2,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription required
Online doctors for IMMUNINE 1200 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR FOR INFUSION
Discuss questions about IMMUNINE 1200 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















