
ILUMETRI 200 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
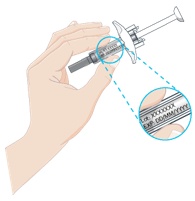

How to use ILUMETRI 200 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ilumetri 200 mg solution for injection in pre-filled syringe
Tildrakizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ilumetri and what is it used for
- What you need to know before you use Ilumetri
- How to use Ilumetri
- Possible side effects
- Storage of Ilumetri
- Contents of the pack and other information
1. What is Ilumetri and what is it used for
Ilumetri contains the active substance tildrakizumab. Tildrakizumab belongs to a group of medicines called interleukin inhibitors (IL).
This medicine works by blocking the activity of a protein called IL-23, a substance that is found in the body and is involved in normal inflammatory and immune responses and is present in large quantities in diseases such as psoriasis.
Ilumetri is used to treat a skin condition called plaque psoriasis in adults with moderate to severe disease.
The use of Ilumetri will benefit you because it improves skin lesions and reduces symptoms.
2. What you need to know before you use Ilumetri
Do not use Ilumetri:
- If you are allergic to tildrakizumab or any of the other ingredients of this medicine (listed in section 6).
- If you have an infection that your doctor considers important, for example, active tuberculosis, which is an infectious disease that mainly affects the lungs.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting treatment with Ilumetri:
- If you have allergic reactions with symptoms such as chest tightness, wheezing, swelling of the face, lips, or throat, do not inject more Ilumetri and contact your doctor immediately.
- If you currently have an infection or if you get long-term or repeated infections.
- If you have been recently vaccinated or are planning to be vaccinated.
If you are not sure if you are in any of the above circumstances, consult your doctor, pharmacist, or nurse before using Ilumetri.
Each time you receive a new pack of Ilumetri, it is important that you note the date and batch number (which appears on the pack after "Batch") and keep this information in a safe place.
Monitoring of infections and allergic reactions
Ilumetri may cause serious side effects, such as infections and allergic reactions. You should be alert to the signs of these conditions while using Ilumetri.
Stop using Ilumetri and inform your doctor or seek medical attention immediately if you notice signs that may indicate a possible serious infection or allergic reaction (see section 4. Possible side effects).
Children and adolescents
Ilumetri is not recommended for use in children and adolescents under 18 years of age, as it has not been evaluated in this patient group.
Other medicines and Ilumetri
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes vaccines and immunosuppressive medicines (medicines that affect the immune system).
Certain types of vaccines (live vaccines) should not be administered while using Ilumetri. There are no data available on the concomitant use of Ilumetri and live vaccines.
Pregnancy, breastfeeding, and fertility
It is recommended to avoid the use of Ilumetri during pregnancy. The effects of this medicine on pregnant women are unknown.
If you are a woman of childbearing age, it is recommended that you do not become pregnant and should use an effective contraceptive method while receiving treatment with Ilumetri and for at least 17 weeks after the end of treatment.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Ilumetri has no or negligible influence on the ability to drive and use machines.
Ilumetri contains polysorbates
This medicine contains 0.5 mg of polysorbate 80 (E 433) in each pre-filled syringe, equivalent to 0.5 mg/ml. Polysorbates may cause allergic reactions. Inform your doctor if you have any known allergy.
3. How to use Ilumetri
Ilumetri should be used under the direction and supervision of a doctor with experience in the diagnosis and treatment of psoriasis.
Follow exactly the administration instructions of this medicine as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again. This medicine is for single use.
The recommended dose of Ilumetri is 100 mg by subcutaneous injection at weeks 0 and 4 and every 12 weeks thereafter.
If you are a patient with a high disease burden or with a body weight over 90 kg, your doctor may recommend a dose of 200 mg.
Your doctor will decide the duration of treatment with Ilumetri.
After you have correctly learned the technique of subcutaneous injection, you can inject Ilumetri yourself if the doctor considers it appropriate.
To consult the instructions on how to inject Ilumetri, read the "Instructions for use" at the end of this leaflet.
Consult your doctor when you will have the injections and follow-up visits.
Use in children and adolescents
The safety and efficacy of Ilumetri in children and adolescents under 18 years of age have not been established, so the use of Ilumetri in children and adolescents is not recommended.
If you use more Ilumetri than you should
If more Ilumetri has been administered than it should or the dose has been administered earlier than indicated by the doctor's prescription, inform your doctor.
If you forget to use Ilumetri
If you have forgotten or missed an injection of Ilumetri, administer the dose as soon as possible. Then, resume administration at the usual interval.
If you stop treatment with Ilumetri
The decision to stop treatment with Ilumetri should be made together with your doctor. It is possible that your symptoms may recur when stopping treatment.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
If you notice any of the following symptoms, contact your doctor immediately:
- Swelling of the face, lips, or throat
- Difficulty breathing
These may be signs of an allergic reaction.
Other side effects
Most of the following side effects are mild. If any of the following side effects gets serious, inform your doctor or pharmacist.
Very common(may affect more than 1 in 10 people)
- Upper respiratory tract infections
Common(may affect up to 1 in 10 people)
- Gastroenteritis
- Nausea
- Diarrhea
- Pain at the injection site
- Back pain
- Headache
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ilumetri
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the pre-filled syringe after EXP. The expiry date is the last day of the month shown.
Store the medicine in the original carton to protect it from light. Do not shake.
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
After removing the pre-filled syringe from the refrigerator, wait for approximately 30 minutes for the Ilumetri solution in the syringe to reach room temperature (maximum 25 °C). Do not heat it in any other way.
Do not use if the liquid contains visible particles, is cloudy, or is clearly brown.
Once removed from the refrigerator, do not store tildrakizumab at more than 25 °C or refrigerate it again.
In the space provided on the outer carton, note the date you removed the medicine from the refrigerator and the disposal date that applies. Use the syringe before 30 days have passed since you removed it from the refrigerator or before the expiry date, whichever comes first.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Ilumetri Composition
- The active ingredient is tildrakizumab. Each pre-filled syringe contains 200 mg of tildrakizumab.
- The other components are L-histidine, L-histidine hydrochloride monohydrate, polysorbate 80 (E 433), sucrose, and water for injectable preparations.
Appearance of Ilumetri and Container Contents
Ilumetri 200 mg solution for injection (injection) in a pre-filled syringe is a clear or slightly opalescent solution, colorless to slightly yellow. Ilumetri 200 mg solution for injection (injection) in a pre-filled syringe is available in unit packs containing 1 pre-filled syringe.
Marketing Authorization Holder
Almirall, S.A.
Ronda General Mitre, 151
08022 Barcelona, Spain
Manufacturer
Industrias Farmacéuticas Almirall, S.A.
Ctra. de Martorell 41-61
08740 Sant Andreu de la Barca, Barcelona, Spain
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien/ Luxembourg/Luxemburg Almirall N.V. Tél/Tel: +32 (0)2 771 86 37 | Italia Almirall SpA Tel: +39 02 346181 |
Eesti/Ελλáδα/ España/ Hrvatska/ Kúπρος/Latvija/Lietuva/ Magyarország/ Malta/ România/ Slovenija Almirall, S.A. Teη./Tel/Τηλ: +34 93 291 30 00 | Nederland Almirall B.V. Tel: +31 (0)30 711 15 10 |
Ceská republika/Slovenská republika Almirall s.r.o Tel: +420 739 686 638 | |
Danmark/ Norge/ Sverige Almirall ApS Tlf./Tel: +45 70 25 75 75 | Österreich Almirall GmbH Tel: +43 (0)1/595 39 60 |
Deutschland Almirall Hermal GmbH Tel: +49 (0)40 72704-0 | Polska Almirall Sp.z o. o. Tel.: +48 22 330 02 57 |
France Almirall SAS Tél: +33(0)1 46 46 19 20 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 4220606 |
Ireland Almirall, S.A. Tel: +353 1800 849322 | Suomi/Finland Orion Pharma Puh/Tel: +358 10 4261 |
Ísland Vistor hf. Sími: +354 535 70 00 |
Date of Last Revision of this Prospectus:06/2024
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website https://www.ema.europa.eu/.
INSTRUCTIONS FOR USE
Before using the pre-filled syringe:
Important Points to Know
- Before using the Ilumetri pre-filled syringe, read and carefully follow all the step-by-step instructions. Save the instructions for use and consult them when needed.
- The pre-filled syringe must not be shaken.
- Read the Ilumetri prospectus for more information about the medicine.
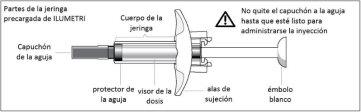
PRODUCT DESCRIPTION
The Ilumetri pre-filled syringe looks like this:
PREPARATION
- Remove the package from the refrigerator (if stored in the refrigerator)
- Make sure the dose on the syringe matches the one prescribed by your doctor.
- Remove the box from the refrigerator and place it unopened on a clean, flat work surface.
 Wait 30 minutes (if stored in the refrigerator)
Wait 30 minutes (if stored in the refrigerator)
- Leave the pre-filled syringe in the outer carton (with the lid closed) at room temperature for 30 minutes.
- Inspect the medicine
- Remove the pre-filled syringe from the carton when you are ready for injection.
- Check the expiration date on the carton and the pre-filled syringe and discard it if the date has passed.
- DO NOT remove the needle cap until you are ready to administer the injection.
- Before administering Ilumetri, visually inspect it for particles and color change.
- Ilumetri is a clear or slightly opalescent solution, colorless to slightly yellow.
- DO NOT use it if the liquid contains visible particles or if the syringe is damaged. It is possible that there may be air bubbles; there is no need to remove them.
- DO NOT use the product if it has fallen on a hard surface or is damaged.
- Gather all the materials you need
- On a clean, well-lit work surface, place the following:
- alcohol swabs
- cotton ball or gauze
- band-aid
- sharps disposal container
- Wash your hands
- Wash your hands well with soap and water.
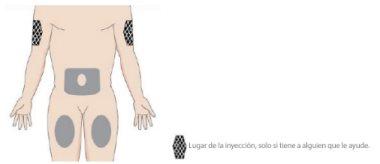
- Choose the injection site
- Choose a place to administer the injection where the skin is healthy and easily accessible, such as the abdomen, thighs, or upper arm.
- DO NOT administer the injection within 5 cm of the navel or in areas of skin with sensitivity, bruises, abnormal redness, hardening, or psoriasis lesions.
- DO NOT inject into scars, stretch marks, or blood vessels.
- The upper arm is only suitable if the injection is administered by another person.
- Alternate injection sites with each administration.
- Clean the injection site
- Clean the injection site with an alcohol swab and let the skin dry.
- Do not touch this area again before administering the injection.
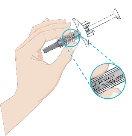
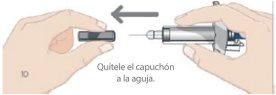
- Remove the needle cap
- While holding the body of the pre-filled syringe, remove the needle cap as shown and discard it. You may see 1 or 2 drops of liquid, which is normal.
- DO NOT touch the white plunger yet.
- DO NOT use the product if the pre-filled syringe or needle is bent.
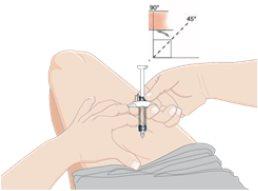
9.Pinch the skin and insert the needle
- Gently pinch the skin at the chosen injection site.
- Insert the entire needle into the pinched skin between your fingers at an angle of 45 to 90 degrees.
- DO NOT place your finger on the plunger while inserting the needle.
- Hold the pre-filled syringe firmly.
10.Give yourself the injection
- Once the needle is inserted, gently release the skin.
- Push the white plunger down until it reaches the stop. This activates a safety mechanism that ensures the complete retraction of the needle after the injection is administered.
- If the white plunger reaches the stop, it cannot be moved further, and there are no spills, a complete dose has been administered.
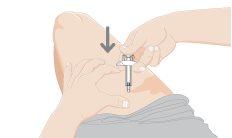
11.Remove the used syringe
- Completely remove the needle from the skin before releasing the white plunger.
- After releasing the white plunger, the safety lock will pull the needle into the needle protector.
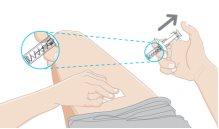
- Discard the used syringe in a sharps disposal container immediately after use.
If there is any residual liquid or a little blood, clean the injection site with a cotton ball or gauze while applying pressure. If necessary, you can use a band-aid to cover the injection site.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ILUMETRI 200 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 100 mgActive substance: tildrakizumabManufacturer: Almirall S.A.Prescription requiredDosage form: INJECTABLE, 100 mgActive substance: tildrakizumabManufacturer: Almirall S.A.Prescription requiredDosage form: INJECTABLE, 100 mgActive substance: tildrakizumabManufacturer: Almirall S.A.Prescription required
Online doctors for ILUMETRI 200 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about ILUMETRI 200 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






