
IKERVIS 1 mg/ml eye drops in emulsion

How to use IKERVIS 1 mg/ml eye drops in emulsion
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
IKERVIS 1 mg/ml Eye Drops, Emulsion
ciclosporin (ciclosporin)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is IKERVIS and what is it used for
- What you need to know before you use IKERVIS
- How to use IKERVIS
- Possible side effects
- Storage of IKERVIS
- Contents of the pack and further information
1. What is IKERVIS and what is it used for
IKERVIS contains the active substance ciclosporin. Ciclosporin belongs to a group of medicines called immunodepressants, which are used to reduce inflammation.
IKERVIS is used to treat adults with severe keratitis (inflammation of the cornea, the transparent layer on the front of the eye). It is used in patients with dry eye syndrome who have not improved despite treatment with artificial tears.
You should consult a doctor if it worsens or does not improve.
You should visit your doctor's office at least every 6 months so that they can evaluate the effect of IKERVIS.
2. What you need to know before you use IKERVIS
Do not use IKERVIS:
- if you are allergic to ciclosporin or any of the other ingredients of this medicine (listed in section 6);
- if you have had or have cancer in or around the eye;
- if you have an eye infection.
Warnings and precautions
Use IKERVIS only as eye drops for the affected eye(s).
Consult your doctor or pharmacist before starting to use IKERVIS:
- if you have had a previous eye infection caused by the herpes virus that may have damaged the transparent front part of the eye (cornea);
- if you are taking any medicine that contains steroids;
- if you are taking any medicine to treat glaucoma.
Contact lenses may increase the damage to the transparent front part of the eye (cornea). Therefore, you should remove your contact lenses when going to bed, before using IKERVIS; you can put them back on when you wake up.
Children and adolescents
IKERVIS should not be used in children and adolescents under 18 years of age.
Other medicines and IKERVIS
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Consult your doctor if you are using a steroid eye drop with IKERVIS, as these may increase the risk of side effects.
You should use the IKERVIS eye drop at least 15 minutesafter using any other eye drop.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not useIKERVIS if you are pregnant.
If you can become pregnant, you should use contraception while using this medicine.
It is likely that there will be very small amounts of IKERVIS in breast milk. If you are breastfeeding, ask your doctor for advice before using this medicine.
Driving and using machines
You may have blurred vision immediately after using the IKERVIS eye drop. Wait until your vision is clear before driving or using machines.
3. How to use IKERVIS
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended doseis one drop in the affected eye(s), once a day at bedtime.
Instructions for use
Follow these instructions carefully and consult your doctor or pharmacist if you have any questions.
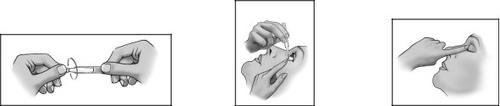
1 2 3
- Wash your hands.
- If you wear contact lenses, remove them when going to bed, before using the eye drop; you can put them back on when you wake up.
- Open the aluminum pouch, which contains 5 single-dose containers.
- Take a single-dose container from the aluminum pouch and.
- Gently shake the single-dose container before use.
- Twist the cap to open it (image 1).
- Lower the lower eyelid with the help of a finger (image 2).
- Tilt your head back and look up at the ceiling.
- Gently squeeze to release one drop of medicine into the eye. Make sure not to touch the eye with the tip of the single-dose container.
- Blink several times to spread the medicine across the eye.
- After using IKERVIS, press the corner of your eye next to your nose and gently close your eyelids for 2 minutes (image 3). This helps prevent IKERVIS from reaching other parts of your body.
- If you use the eye drop in both eyes, repeat these steps in the other eye.
- Discard the single-dose container after use, even if there is still some liquid left in it.
- You should keep the remaining single-dose containers in the aluminum pouch.
If the drop falls outside the eye, try again.
If you use more IKERVIS than you should, rinse your eye with water. Do not apply more drops until the next scheduled dose.
If you forget to use IKERVIS, continue with the next scheduled dose.Do not apply a double dose to make up for forgotten doses. Do not use more than one drop per day in the affected eye(s).
If you stop treatment with IKERVISwithout consulting your doctor, the inflammation of the transparent front part of the eye (known as keratitis) will not be controlled and may lead to visual impairment.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported:
The most common side effects occur in and around the eyes.
Very common (may affect more than 1 in 10 people)
Pain when applying the drops to the eye.
Common (may affect up to 1 in 10 people)
Irritation, redness, and increased tearing when applying the drops to the eye, redness of the eyelid, watery eyes, redness of the eye, blurred vision. Swelling of the eyelid, redness of the conjunctiva (thin membrane covering the front of the eye), eye irritation, eye pain.
Uncommon (may affect up to 1 in 100 people)
Uncommon side effects related to the eye:
Discomfort, itching, or irritation in or around the eye, including the feeling of having something in it. Irritation or swelling of the conjunctiva (thin membrane covering the front of the eye), eye allergy, changes in tear production, eye discharge, inflammation of the iris (colored part of the eye) or eyelid, deposits in the eye, bacterial infection or inflammation of the cornea (transparent front part of the eye), abrasion on the outer layer of the cornea, white spots on the cornea, cyst on the eyelid, itching of the eyelid, painful skin rash around the eye caused by the herpes zoster virus.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of IKERVIS
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer carton, aluminum pouch, and single-dose containers after ‘EXP’. The expiry date is the last day of the month stated.
Do not freeze.
After opening the aluminum pouches, the single-dose containers should be kept in them to protect them from light and to prevent evaporation. Discard any opened single-dose container with remaining emulsion immediately after use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of IKERVIS
- The active substance is ciclosporin. One milliliter of IKERVIS contains 1 mg of ciclosporin.
- The other ingredients are medium-chain triglycerides, cetalkonium chloride, glycerol, tyloxapol, poloxamer 188, sodium hydroxide (for pH adjustment), and water for injections.
Appearance and pack contents
IKERVIS is a white, milky eye drop emulsion.
It is supplied in single-dose containers made of low-density polyethylene (LDPE).
Each single-dose container contains 0.3 ml of eye drop emulsion.
The single-dose containers are packaged in a sealed aluminum pouch.
Pack sizes: 30 and 90 single-dose containers.
Not all pack sizes may be marketed.
Marketing authorisation holder
SANTEN Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer
EXCELVISION
Rue de la Lombardière
ZI la Lombardière
F-07100 Annonay
France
SANTEN Oy
Niittyhaankatu 20
33720 Tampere
Finland
For further information about this medicine, please contact the local representative of the marketing authorisation holder:
België/Belgique/BelgienLietuva
Santen Oy Santen Oy
Tél/Tel: +32 (0) 24019172 Tel: +370 37 366628
????????Luxembourg/Luxemburg
Santen Oy Santen Oy
Тел.: +359 (0) 888 755 393 Tél/Tel: +352 (0) 27862006
Česká republikaMagyarország
Santen Oy Santen Oy
Tel: +420 234 102 170 Tel.: +36 (06) 16777305
DanmarkMalta
SantenPharma AB Santen Oy
Tlf: +45 78737843 Tel: +358 (0) 3 284 8111
DeutschlandNederland
Santen GmbH Santen Oy
Tel: +49 (0) 3030809610 Tel: +31 (0) 207139206
EestiNorge
Santen Oy SantenPharma AB
Tel: +372 5067559 Tlf: +47 21939612
ΕλλάδαÖsterreich
Santen Oy Santen Oy
Τηλ: +358 (0) 3 284 8111 Tel: +43 (0) 720116199
EspañaPolska
Santen Pharmaceutical Spain S.L. Santen Oy
Tel: +34 914 142 485 Tel.: +48 (0) 221168608
FrancePortugal
Santen Santen Oy
Tél: +33 (0) 1 70 75 26 84 Tel: +351 308 805 912
HrvatskaRomânia
Santen Oy Santen Oy
Tel: +358 (0) 3 284 8111 Tel: +40 (0) 316300603
IrelandSlovenija
Santen Oy Santen Oy
Tel: +353 (0) 16950008 Tel: +358 (0) 3 284 8111
ÍslandSlovenská republika
Santen Oy Santen Oy
Sími: +358 (0) 3 284 8111 Tel: +421 (01) 23 332 5519
ItaliaSuomi/Finland
Santen Italy S.r.l. Santen Oy
Tel: +39 0236009983 Puh/Tel: +358 (0) 974790211
ΚύπροςSverige
Santen Oy SantenPharma AB
Τηλ: +358 (0) 3 284 8111 Tel: +46 (0) 850598833
LatvijaUnited Kingdom
Santen Oy Santen UK Limited
Tel: +371 677 917 80 Tel: +44 (0) 845 075 4863
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IKERVIS 1 mg/ml eye drops in emulsionDosage form: EYE DROP, 0.9 mg/mlActive substance: ciclosporinManufacturer: Sun Pharmaceutical Industries (Europe) B.V.Prescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: ciclosporinManufacturer: Santen OyPrescription requiredDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not required
Online doctors for IKERVIS 1 mg/ml eye drops in emulsion
Discuss questions about IKERVIS 1 mg/ml eye drops in emulsion, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















