
CEQUA 0.9 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS

How to use CEQUA 0.9 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information Leaflet
Cequa 0.9 mg/ml eye drops, solution in single-dose container
ciclosporin
Read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Cequa is and what it is used for
- What you need to know before you use Cequa
- How to use Cequa
- Possible side effects
- Storage of Cequa
- Contents of the pack and other information
1. What Cequa is and what it is used for
Cequa contains the active substance ciclosporin. Ciclosporin belongs to a group of medicines called immunosuppressants, which are used to reduce inflammation.
Ciclosporin is used to treat moderate to severe dry eye disease (keratoconjunctivitis sicca) in adult patients who have not responded adequately to artificial tears.
You should consult a doctor if it gets worse or does not improve.
You should visit your doctor's office at least every 3 months for your doctor to assess the effect of this medicine.
2. What you need to know before you use Cequa
Do not use Cequa:
- if you are allergic to ciclosporin or any of the other ingredients of this medicine (listed in section 6);
- if you have had or have cancer in or around the eye;
- if you have an eye infection.
Warnings and precautions
Use this medicine only as eye drops for the (affected) eye(s).
Consult your doctor or pharmacist before you start using this medicine:
- if you have had a previous eye infection with the herpes virus that may have damaged the clear front part of the eye (cornea);
- if you are taking any medicine that contains steroids;
- if you are taking any medicine to treat glaucoma.
You should remove your contact lenses before using this medicine; you can put them back 15 minutes after administration of this medicine.
Children and adolescents
This medicine must not be used in children and adolescents under 18 years.
Other medicines and Cequa
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Consult your doctor if you are using an eye drop that contains steroids, as these may increase the risk of side effects.
This medicine should be used at least 15 minutes after using any other eye drop.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
You must not use this medicine if you are pregnant.
If you can become pregnant, you must use contraception while using this medicine.
It is possible that very small amounts of ciclosporin are present in breast milk. If you are breast-feeding, consult your doctor before using this medicine.
Driving and using machines
You may have blurred vision immediately after using this medicine. In this case, wait until your vision is clear before driving or using machines.
Cequa contains phosphates
This medicine contains 0.0159 mg of phosphates in each drop. If you have severe damage to the clear layer on the front of the eye (cornea), treatment with phosphates, in very rare cases, may cause cloudy patches on the cornea due to calcium.
3. How to use Cequa
Follow exactly the instructions of administration of this medicine given to you by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again.
The recommended dose is one drop in each affected eye, twice a day, with an approximate interval of 12 hours.
Instructions for use

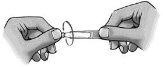
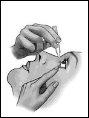 Follow these instructions carefully and consult your doctor or pharmacist if there is anything you do not understand.
Follow these instructions carefully and consult your doctor or pharmacist if there is anything you do not understand.
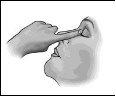
1 2 3
- Wash your hands.
- If you wear contact lenses, remove them before using the eye drops; you can put them back 15 minutes after the drops have been administered.
- Open the aluminum bag, remove a single-dose container, and put the remaining single-dose containers back inside the aluminum bag.
- Turn the cap (figure 1).
- Lower the lower eyelid (figure 2).
- Tilt your head back and look up at the ceiling.
- Gently squeeze to release one drop of medicine into the eye. Make sure not to touch the eye with the tip of the single-dose container.
- Blink several times to spread the drop in the eye.
- After applying the drop, press the corner of your eye next to your nose and gently close your eyelids for 2 minutes (figure 3)(this helps the local effect).
- If you use the eye drops in both eyes, repeat these steps in the other eye.
- Discard the single-dose container as soon as you have used it, even if there is still some medicine left in it.
- You should keep the remaining single-dose containers in the aluminum bag.
If the drop falls outside the eye, try again.
If you use more Cequa than you should, rinse your eye with water. Do not apply more drops until it is time for your next dose.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Cequa, continue with the next scheduled dose.
Do not take a double dose to make up for forgotten doses. Do not use more than one drop at a time in the affected eye(s).
If you stop using Cequa, consult your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed:
The most common side effects occur in and around the eyes.
Very common (may affect more than 1 in 10 people): pain or discomfort when a drop is applied for the first time.
Common (may affect up to 1 in 10 people): redness of the eye, irritation of the eye, watery eyes, irritation in the eye.
Uncommon (may affect up to 1 in 100 people): increased tearing of the eye, increased sensitivity to light, eye pain, swelling of the conjunctiva (the skin that covers the white part of the eye), inflammation of the eyelid margins, eye and periocular infections, headache, and throat irritation.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines and Healthcare Products Agency (AEMPS) website: www.notificaRAM. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cequa
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, the aluminum bag, and the single-dose containers after “EXP”. The expiry date is the last day of the month shown.
Do not store above 25°C. Do not freeze. After opening the aluminum bags, the single-dose containers should be kept in them to avoid evaporation. Shelf-life after opening of the aluminum bag: 5 days. Discard immediately after use any opened single-dose container with remaining solution.
Do not use this medicine if you notice visible particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Cequa contains
- The active substance is ciclosporin. One milliliter of Cequa contains 0.9 mg of ciclosporin.
- The other ingredients are: macrogolglycerol hydroxystearate, octoxinol-40, sodium dihydrogen phosphate dihydrate (E339), disodium phosphate anhydrous (E339), sodium chloride, povidone (E1201), hydrochloric acid (for pH adjustment) (E507), sodium hydroxide (for pH adjustment) (E524), and water for injections.
Appearance and packaging
Cequa is a clear, colorless solution in single-dose containers.
It is supplied in low-density polyethylene (LDPE) single-dose containers.
Each single-dose container contains 0.25 ml of eye drops solution.
The single-dose containers are packaged in a sealed aluminum bag with a polyethylene coating.
Container sizes: containers with 60 or 180 single-dose containers, or a multiple container containing 180 units (3 cardboard boxes with 60 single-dose containers each).
Not all container sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Sun Pharmaceutical Industries BV
Polarisavenue 87,
2132JH Hoofddorp,
Netherlands
Manufacturer
Laboratoire Unither 1 Rue De L’Arquerie Coutances 50200 France |
You can obtain further information on this medicine by contacting the local representative of the marketing authorization holder:
Sun Pharma Laboratorios, S.L.
Rambla de Catalunya 53-55
08007 Barcelona
Spain
Phone: +34 93 342 78 90
This medicine is authorized in the Member States of the European Economic Area under the following names:
Netherlands: Cequa 0.9 mg/ml oogdruppels, oplossing in verpakking voor eenmalig gebruik
Austria: Cequa 0,9 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis
Germany: Cequa 0,9 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis
Denmark: Sekua 0.9 mg/ml øjendråber, opløsning i enkeltdosisbeholder |
Spain: Cequa 0,9 mg/ml colirio en solución en envase unidosis
Finland: Cequa 0.9 mg/ml silmätipat, liuos kerta-annospakkauksessa | |
France: Cequa 0,9 mg/mL, collyre en solution en récipient unidose Italy: Cequa
| |
Sweden: Cequa 0.9 mg/ml ögondroppar, lösning i endosbehållare |
Date of last revision of this leaflet: March 2024
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CEQUA 0.9 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 1 mg/mlActive substance: ciclosporinManufacturer: Santen OyPrescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: ciclosporinManufacturer: Santen OyPrescription requiredDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not required
Online doctors for CEQUA 0.9 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS
Discuss questions about CEQUA 0.9 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















