
IDELVION 250 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use IDELVION 250 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
IDELVION 250 IU, powder and solvent for solution for injection
IDELVION 500 IU, powder and solvent for solution for injection
IDELVION 1000 IU, powder and solvent for solution for injection
IDELVION 2000 IU, powder and solvent for solution for injection
IDELVION 3500 IU, powder and solvent for solution for injection
albutrepenonacog alfa (recombinant coagulation factor IX)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is IDELVION and what is it used for
- What you need to know before you use IDELVION
- How to use IDELVION
- Possible side effects
- Storage of IDELVION
- Contents of the pack and further information
1. What is IDELVION and what is it used for
What is IDELVION?
IDELVION is a medicine used to treat hemophilia by replacing the natural factor IX clotting factor. The active substance of IDELVION is albutrepenonacog alfa (recombinant fusion protein linking factor IX clotting factor with albumin [rIX-FP]).
Factor IX is involved in blood clotting. Patients with hemophilia B do not have this factor, which means that their blood does not clot as quickly as it should, resulting in a greater tendency to bleed. IDELVION works by replacing factor IX in patients with hemophilia B to make their blood clot.
What is IDELVION used for?
IDELVION is used to prevent or stop bleeding caused by a lack of sufficient factor IX in patients of all ages with hemophilia B (also known as congenital factor IX deficiency or Christmas disease).
2. What you need to know before you use IDELVION
Do not use IDELVION
- if you are allergic to the active substance (albutrepenonacog alfa) or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to hamster proteins.
Warnings and precautions
It is strongly recommended that each time you use IDELVION, you record the name and batch number of the product to track the products and batches of the product you have used.
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the administered product must be clearly recorded.
Consult your doctor, pharmacist, or nurse before starting to use IDELVION.
- It is possible that allergic reactions (hypersensitivity) may occur. The product contains residual hamster proteins (see also "Do not use IDELVION"). If symptoms of allergic reactions occur, you must stop treatment immediately and contact your doctor or treatment center where you are being monitored. Your doctor should inform you of the first signs of hypersensitivity reactions.These include hives, widespread skin rash, chest pressure, difficulty breathing, low blood pressure (hypotension), and anaphylaxis (a severe allergic reaction that causes severe breathing difficulties or dizziness).
- Due to the risk of allergic reactions with factor IX, the initial administration of IDELVION should be performed under medical supervision to ensure access to adequate medical care in case of allergic reactions.
- The formation of inhibitors(neutralizing antibodies) is a known complication that has been reported during treatment with IDELVION. Inhibitors prevent the treatment from working properly. If IDELVION does not control your bleeding, inform your doctor immediately. You should be regularly monitored for the development of inhibitors.
- If you have liver or heart disease or have recently had major surgery, please inform your doctor, as there is a higher risk of blood clotting complications.
- If a central venous access device (CVAD) is needed for the administration of IDELVION, your doctor will consider the risk of CVAD-related complications, such as local infections, bacteria in the blood (bacteremia), and the formation of a blood clot in the blood vessels (thrombosis) at the catheter insertion site.
Using IDELVION with other medicines
- Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
- During pregnancy and breastfeeding, IDELVION should only be administered if it is clearly necessary.
Driving and using machines
IDELVION does not affect your ability to drive or use machines.
IDELVION contains sodium
This medicine contains up to 8.6 mg of sodium (main component of cooking/table salt) in each vial. This is equivalent to 0.4% of the maximum recommended daily intake of sodium for an adult.
3. How to use IDELVION
Your treatment should be started and supervised by a doctor with experience in the treatment of blood clotting disorders. Follow your doctor's instructions exactly for the use of this medicine. Consult your doctor if you have any doubts.
Your doctor will calculate the dose of IDELVION you need. The amount of IDELVION you need and the duration of treatment depend on:
- the severity of your disease
- the location and intensity of the bleeding
- your clinical condition and clinical response
- your body weight
IDELVION is administered as an injection into a vein (intravenous, IV) after reconstitution of the powder with the solvent provided by your doctor or nurse. You or another person may also administer IDELVION as an intravenous injection, but only after receiving proper training.
If you use more IDELVION than you should
Contact your doctor immediately if you inject more IDELVION than your doctor has recommended.
If you stop treatment with IDELVION
Do not stop using IDELVION without consulting your doctor first.
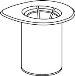
Reconstitution and administration
General instructions
- The powder must be mixed with the solvent (liquid) and withdrawn from the vial while keeping the medicine sterile (germ-free). Your doctor will show you how to prepare the solution and how to withdraw the solution from the vial correctly.
- IDELVION must not be mixed with other medicines or solvents, except those mentioned in section 6.
- The solution should be clear or slightly opalescent, between yellow and colorless, i.e., it may shine when exposed to light but must not contain any visible particles. After withdrawal or filtration of the solution (see below), it should be visually inspected before use. Do not use the solution if it is cloudy or contains flakes or particles.
- Disposal of unused product and all residual materials will be carried out in accordance with local regulations and your doctor's instructions.
Reconstitution
Without opening any of the vials, warm the IDELVION powder and liquid to room temperature or body temperature. This can be done by leaving the vials at room temperature for about an hour or by holding them in your hands for a few minutes.
DO NOT expose the vials to direct heat. The vials should not be heated above body temperature (37°C).
Carefully remove the protective caps from the vials and then clean the exposed part of the rubber stoppers with an alcohol swab. Allow the vials to dry before opening the Mix2Vial package (which contains the transfer device with filter) and then follow the instructions below.
|
|
|
|
|
|
|
|
|
Discard the solvent vial with the blue Mix2Vial adapter attached. |
|
|
|
|
Transfer and administration
|
|
9 |
|
Use the venipuncture kit provided with the product and insert the needle into a vein. Allow the blood to flow to the end of the tube. Connect the syringe to the threaded end of the venipuncture kit. Slowly inject the reconstituted solution (at a speed that is comfortable for you, up to a maximum of 5 ml/min) into the veinaccording to the instructions given by your doctor. Try to prevent blood from entering the syringe containing the product.
Check if you experience side effects immediately after injection. If you experience any side effect that may be related to the administration of IDELVION, the injection should be interrupted (see also sections 2 and 4).
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Contact your doctor:
The following side effects have been observed with factor IX medicines:
- It is possible that allergic reactions of an allergic type (frequent) may occur, including symptoms such as redness, itching of the skin (generalized urticaria), chest pressure, difficulty breathing, low blood pressure (hypotension), and anaphylaxis (a severe reaction that causes severe breathing difficulties and dizziness). If this happens, you must stop the administration of the medicine immediately and contact your doctor.
- Inhibitors: the medicine stops working properly (continuous bleeding). You may develop an inhibitor (neutralizing antibody) of factor IX (frequency unknown), which means that factor IX will no longer work properly. If this happens, you must stop the administration of the medicine immediately and contact your doctor.
The following side effects have been observed frequentlywith IDELVION (may affect up to 1 in 10 people):
- Headache
- Injection site reactions
- Dizziness
- Skin rash
The following side effects have been observed infrequently(may affect up to 1 in 100 people):
- Eczema
Side effects in children and adolescents
It is expected that the side effects in children will be the same as in adults.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that the side effects are not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of IDELVION
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date stated on the label and carton.
- Do not store above 25°C.
- Do not freeze.
- Keep the vial in its carton to protect it from light.
- Once the product is reconstituted, it should be used immediately.
If the reconstituted product is not administered immediately, the storage times and conditions before use are the responsibility of the user.
6. Container Content and Additional Information
IDELVION Composition
The active ingredient is:
250 IU per vial; after reconstitution with 2.5 ml of water for injectable preparations, the solution contains 100 IU/ml of albutrepenonacog alfa.
500 IU per vial; after reconstitution with 2.5 ml of water for injectable preparations, the solution contains 200 IU/ml of albutrepenonacog alfa.
1,000 IU per vial; after reconstitution with 2.5 ml of water for injectable preparations, the solution contains 400 IU/ml of albutrepenonacog alfa.
2,000 IU per vial; after reconstitution with 5 ml of water for injectable preparations, the solution contains 400 IU/ml of albutrepenonacog alfa.
3,500 IU per vial; after reconstitution with 5 ml of water for injectable preparations, the solution contains 700 IU/ml of albutrepenonacog alfa.
The other components are:
Sodium citrate, polysorbate 80, mannitol, sucrose, and hydrochloric acid (for pH adjustment).
See the last paragraph of section 2.
Solvent: water for injectable preparations
Appearance of IDELVION and Container Content
IDELVION is presented as a yellowish-white powder and is supplied with a solvent in the form of water for injectable preparations.
The reconstituted solution should be transparent or slightly opalescent, yellowish to colorless, i.e., it may shine when exposed to light but should not contain any visible particles.
Presentation
A container with 250, 500, or 1,000 IU containing:
1 vial with powder
1 vial with 2.5 ml of water for injectable preparations
1 transfer device with a 20/20 filter
Administration equipment (inner box):
1 disposable 5 ml syringe
1 venipuncture device
2 alcohol-impregnated swabs
1 non-sterile dressing
A container with 2,000 or 3,500 IU containing:
1 vial with powder
1 vial with 5 ml of water for injectable preparations
1 transfer device with a 20/20 filter
Administration equipment (inner box):
1 disposable 10 ml syringe
1 venipuncture device
2 alcohol-impregnated swabs
1 non-sterile dressing
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
CSL Behring GmbH
Emil-von-Behring-Straße 76
35041 Marburg
Germany
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien CSL Behring NV Tel: +32 15 28 89 20 | Lithuania CentralPharma Communications UAB Tel: +370 5 243 0444 |
| Luxembourg/Luxemburg CSL Behring NV Tel: +32 15 28 89 20 |
Czech Republic CSL Behring s.r.o. Tel: +420 702 137 233 | Hungary CSL Behring Kft. Tel: +36 1 213 4290 |
Denmark CSL Behring AB Tel: +46 8 544 966 70 | Malta AM Mangion Ltd. Tel: +356 2397 6333 |
Germany CSL Behring GmbH Tel: +49 6190 75 84810 | Netherlands CSL Behring BV Tel: +31 85 111 96 00 |
Estonia CentralPharma Communications OÜ Tel: +372 601 5540 | Norway CSL Behring AB Tel: +46 8 544 966 70 |
Greece CSL Behring ΕΠΕ Tel: +30 210 7255 660 | Austria CSL Behring GmbH Tel: +43 1 80101 1040 |
Spain CSL Behring S.A. Tel: +34 933 67 1870 | Poland CSL Behring Sp.z o.o. Tel: +48 22 213 22 65 |
France CSL Behring S.A. Tel: +33 –(0)-1 53 58 54 00 | Portugal CSL Behring Lda Tel: +351 21 782 62 30 |
Croatia Marti Farm d.o.o. Tel: +385 1 558 8297 | Romania Prisum Healthcare S.R.L. Tel: +40 21 322 0171 |
Ireland CSL Behring GmbH Tel: +49 6190 75 84700 Iceland CSL Behring AB Tel: +46 8 544 966 70 | Slovenia Emmes Biopharma Global s.r.o. podružnica v Sloveniji Tel: +386 41 42 0002 Slovakia CSL Behring Slovakia s.r.o. Tel: +421 911 653 862 |
Italy CSL Behring S.p.A. Tel: +39 02 34964 200 | Finland CSL Behring AB Tel: +46 8 544 966 70 |
Cyprus CSL Behring ΕΠΕ Tel: +30 210 7255 660 | Sweden CSL Behring AB Tel: +46 8 544 966 70 |
Latvia CentralPharma Communications SIA Tel: +371 6 7450497 | |
Date of Last Revision of this Leaflet
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
---------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Dosage
The dose and duration of replacement treatment depend on the severity of the factor IX deficiency, the location and severity of the bleeding, and the patient's clinical condition.
The number of units of factor IX administered is expressed in International Units (IU), in relation to the current WHO standard for products containing factor IX. The plasma activity of factor IX is expressed as a percentage (in relation to normal human plasma) or in International Units (in relation to an international standard for plasma factor IX).
One International Unit (IU) of factor IX activity is equivalent to the amount of factor IX present in 1 ml of normal human plasma.
On-demand treatment
The calculation of the required dose of factor IX is based on the empirical finding that 1 IU of factor IX per kg of body weight increases the plasma activity of factor IX by 1.3 IU/dl on average (1.3% of normal activity) in patients ≥ 12 years and by 1.0 IU/dl (1.0% of normal activity) in patients <12 years. the required dose is determined using following formula:< p>
Required dose (IU) = body weight (kg) x desired increase in factor IX (% of normal level or IU/dl) x (observed recovery reciprocal (IU/kg per IU/dl))
Expected increase in factor IX (IU/dl or % of normal level) = dose (IU) x recovery (IU/dl per IU/kg) / body weight (kg)
The dose and frequency of administration will always be determined based on the observed clinical efficacy in each case.
Patient <12 years of age< em>
In the case of an incremental recovery of 1 IU/dl per 1 IU/kg, the dose is calculated as follows:
Required dose (IU) = body weight (kg) x desired increase in factor IX (IU/dl) x 1 dl/kg
Example:
- A level of 50% of normal is required in a patient with severe hemophilia B weighing 20 kg. The suitable dose would be 20 kg x 50 IU/dl x 1 dl/kg = 1,000 IU.
- It can be expected that a dose of 1,000 IU of IDELVION, administered to a patient weighing 25 kg, will cause a maximum increase in factor IX after injection of 1,000 IU/25 kg x 1.0 (IU/dl per IU/kg) = 40 IU/dl (40% of normal level).
Patient ≥ 12 years of age
In the case of an incremental recovery of 1.3 IU/dl per 1 IU/kg, the dose is calculated as follows:
Required dose (IU) = body weight (kg) x desired increase in factor IX (IU/dl) x 0.77 dl/kg
Example:
- A level of 50% of normal is required in a patient with severe hemophilia B weighing 80 kg. The suitable dose would be 80 kg x 50 IU/dl x 0.77 dl/kg = 3,080 IU.
- It can be expected that a dose of 2,000 IU of IDELVION, administered to a patient weighing 80 kg, will cause a maximum increase in factor IX after injection of 2,000 IU x 1.3 (IU/dl per IU/kg)/80 kg = 32.5 IU/dl (32.5% of normal level).
In the following bleeding events, the activity of factor IX should not be lower than the established plasma activity level (in % of normal or IU/dl) during the corresponding period. The following table can be used as a dosage guide in bleeding episodes and surgery:
Severity of bleeding/ type of surgical procedure | Required factor IX level (% or IU/dl) | Dosing frequency (hours)/duration of treatment (days) |
Bleeding Mild or moderate hemarthrosis, muscle bleeding (except in the iliopsoas) or bleeding in the oral cavity | 30-60 | A single dose should be sufficient in most bleeding episodes. A maintenance dose should be administered after 24 - 72 hours if further evidence of bleeding is observed. |
Major bleeding Potentially life-threatening bleeding, deep muscle bleeding, including the iliopsoas | 60-100 | Should be repeated every 24 - 72 hours during the first week and then a maintenance dose should be administered every week until bleeding stops and the wound heals. |
Minor surgery For example, (including uncomplicated dental extractions) | 50-80 (pre- and postoperative) | A single dose should be sufficient in most minor interventions. If necessary, a maintenance dose can be administered after 24 - 72 hours until bleeding stops and the wound heals. |
Major surgery | 60-100 (pre- and postoperative) | Should be repeated every 24 - 72 hours during the first week and then a maintenance dose should be administered 1 - 2 times a week until bleeding stops and the wound heals. |
Prophylactic treatment
For long-term prophylaxis to prevent bleeding in patients with severe hemophilia B, the usual dose is 35 to 50 IU/kg once a week. Patients well-controlled on a once-weekly regimen may be treated with up to 75 IU/kg every 10 or 14 days. In patients > 18 years, a longer treatment interval may be considered.
In some cases, especially in young patients, it may be necessary to shorten the administration intervals or use higher doses.
After a bleeding episode during prophylaxis, patients should maintain their prophylaxis regimen as much as possible, with administration of 2 doses of IDELVION with a minimum of 24 hours between them, but longer when deemed appropriate for the patient.
Pediatric population
In long-term prophylactic treatment, the recommended dosing regimen is 35 to 50 IU/kg once a week. For adolescents 12 years or older, the dosage recommendations are the same as for adults (see above).
Special warnings and precautions for use
Inhibitors
After repeated treatment with human coagulation factor IX products, patients should be monitored for the development of neutralizing antibodies (inhibitors) that should be quantified in Bethesda Units (BU) using appropriate biological tests.
Cases have been reported in the literature demonstrating a correlation between the appearance of a factor IX inhibitor and allergic reactions. Therefore, patients who experience allergic reactions should be evaluated for the presence of an inhibitor. It should be noted that patients with factor IX inhibitors may be at increased risk of anaphylaxis with subsequent exposure to factor IX.
Treatment monitoring
During the course of treatment, it is recommended to adequately monitor factor IX levels to determine the dose to be administered and the frequency of infusions. Patient responses to factor IX may vary, which demonstrates that they have different half-lives and recoveries. The dose based on body weight may need to be adjusted in patients with insufficient or excessive weight. In the special case of major surgical interventions, it is essential to precisely monitor replacement treatment using coagulation tests (plasma factor IX activity).
When using a one-stage coagulation assay based on activated partial thromboplastin time (APTT) in vitroto determine the activity of factor IX in patient blood samples, the results of plasma factor IX activity may be significantly affected by the APTT reagent and reference standard used in the assay. It is likely that measurement with a one-stage coagulation assay using a kaolin-based APTT reagent or an APTT reagent with Actin FS will result in underestimation of the activity level. This is especially important when changing the laboratory or reagents used in the assay.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IDELVION 250 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 2,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription required
Online doctors for IDELVION 250 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about IDELVION 250 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




 1
1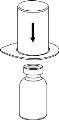 2
2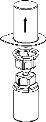 3
3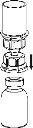 4
4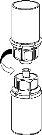 5
5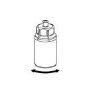 6
6 7
7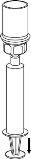 8
8











