
HYCAMTIN 0.25 mg HARD CAPSULES

How to use HYCAMTIN 0.25 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Hycamtin 0.25mg hard capsules
Hycamtin 1mg hard capsules
topotecan
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Hycamtin and what is it used for
- What you need to know before you take Hycamtin
- How to take Hycamtin
- Possible side effects
- Storage of Hycamtin
- Contents of the pack and other information
1. What is Hycamtin and what is it used for
Hycamtin helps to eliminate tumors.
Hycamtin is used to treat small cell lung cancerthat has come back after chemotherapy.
Your doctor will decide with you whether treatment with Hycamtin is better than continuing your initial chemotherapy.
2. What you need to know before you take Hycamtin
Do not take Hycamtin
- if you are allergic to topotecan or any of the other ingredients of this medicine (listed in section 6).
- if you are breast-feeding.
- if your blood cell count is very low.Your doctor will tell you if this is the case, based on your latest blood test results.
Tell your doctorif you are in any of these situations.
Warnings and precautions
Before starting treatment with this medicine, your doctor needs to know:
- if you have liver or kidney problems. It may be necessary to adjust your dose of Hycamtin.
- if you are pregnant or think you may be pregnant. See the section “Pregnancy and breast-feeding” below.
- if you are planning to become a father. See the section “Pregnancy and breast-feeding” below.
Tell your doctorif you are in any of these situations.
Other medicines and Hycamtin
Tell your doctor if you are taking, have recently taken or might take any other medicines, including those obtained without a prescription or any herbal medicines.
There may be a greater chance of you suffering side effects if you are also being treated with ciclosporin A. You will be monitored while taking these two medicines.
Remember to tell your doctor if you start taking any medicine while being treated with Hycamtin.
Pregnancy and breast-feeding
Hycamtin is not recommended during pregnancy. It may harm the fetus, before, during or shortly after treatment. You must use an effective method of contraception. Do not try to become pregnant or father a child until your doctor tells you it is safe to do so.
Men who wish to become fathers should seek advice from their doctor about family planning. If your partner becomes pregnant during your treatment, tell your doctor immediately.
Avoid breast-feeding while being treated with Hycamtin. Do not restart breast-feeding until your doctor tells you it is safe to do so.
Driving and using machines
Hycamtin may cause fatigue. If you feel tired or weak, do not drive or operate machinery.
Important information about some of the ingredients of Hycamtin
This medicine contains small amounts of ethanol (alcohol).
3. How to take Hycamtin
Follow exactly the instructions for administration of this medicine given by your doctor. If you are unsure, consult your doctor or pharmacist again.
The capsule(s) should be swallowed whole, and not chewed, crushed or divided.
The dose (and the number of capsules) of Hycamtin you receive will be calculated by your doctor based on:
- your body size (body surface area measured in square meters)
- the results of your blood tests before treatment
The prescribed number of capsules should be swallowed whole, once a day for five days.
The Hycamtin capsules should not be opened or crushed.If the capsules are pierced or leaking, you should wash your hands immediately with soap and water. If the capsule contents come into contact with your eyes, rinse them immediately with plenty of water for at least 15 minutes. Consult your doctor after eye contact or if you experience a skin reaction.Removing a capsule
These capsules come in a special container to prevent children from removing them.
- Separate a capsule: tear along the perforated lines to separate a blister from the strip.
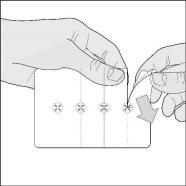
- Remove the outer foil: lift and remove the foil that covers the blister, starting from the colored corner.
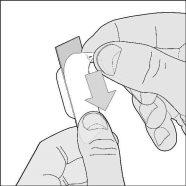
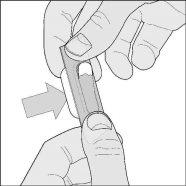
- Remove the capsule: carefully push one end of the capsule along the aluminum foil.
If you take more Hycamtin than you should
If you have taken too many capsules or if a child has taken the medicine by mistake, consult your doctor or pharmacist immediately.
If you forget to take Hycamtin
Do not take a double dose to make up for forgotten doses. Take the next dose at the scheduled time.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects: tell your doctor
These very commonside effects may affect more than 1 in 10 peopletreated with Hycamtin:
- Signs of infection:Hycamtin may reduce the number of white blood cells and decrease your resistance to infections. This can be life-threatening. Some signs of infection are:
- fever
- severe deterioration of your general condition
- local symptoms such as sore throat or urinary problems (e.g. burning sensation when urinating, which may be due to a urinary tract infection).
- Diarrhea.It can be severe. Contact your doctor immediately if you have more than 3 episodes of diarrhea per day.
- Occasionally, the presence of severe stomach pain, fever, and possible diarrhea (rarely with blood) can be signs of intestinal inflammation (colitis)
This rareside effect may affect up to 1 in 1,000 peopletreated with Hycamtin.
- Lung inflammation(interstitial lung disease): you are at greater risk if you already have a lung disease, have received radiation treatment to your lungs, or have taken medicines that have previously damaged your lungs. The signs include:
- difficulty breathing
- cough
- fever.
Tell your doctor immediatelyif you notice any of these symptoms, as you may need to be hospitalized.
Very common side effects
These may affect more than 1 in 10 peopletreated with Hycamtin:
- Feeling of general weakness and tiredness (temporary anemia). In some cases, you may need to have a blood transfusion.
- Bruising or unexplained bleeding, caused by a decrease in the number of cells involved in blood clotting. This can lead to severe bleeding from relatively small wounds, such as small cuts. Rarely, this can lead to more severe bleeding (hemorrhage). Talk to your doctor for advice on how to minimize the risk of bleeding.
- Weight loss and loss of appetite (anorexia); tiredness; weakness.
- Nausea, vomiting.
- Hair loss.
Common side effects
These may affect up to 1 in 10 peopletreated with Hycamtin:
- Allergic reactions or hypersensitivity reactions (including skin rash).
- Inflammation and ulcers in the mouth, tongue, and gums.
- Fever.
- Stomach pain, constipation, indigestion.
- Discomfort.
- Feeling of itching.
Uncommon side effects
These may affect up to 1 in 100 peopletreated with Hycamtin:
- Yellowing of the skin.
Rare side effects
These may affect up to 1 in 1,000 peopletreated with Hycamtin:
- Severe allergic reactions or anaphylactic reactions.
- Swelling caused by fluid retention (angioedema).
- Itchy skin rash (or hives).
Side effects with unknown frequency
The frequency of some side effects cannot be estimated from the available data (effects from spontaneous reports and the frequency cannot be estimated from the available data):
- Severe stomach pain, nausea, vomiting with blood, black or bloody stools (possible symptoms of gastrointestinal perforation).
- Sores in the mouth, difficulty swallowing, abdominal pain, nausea, vomiting, diarrhea, bloody stools (possible signs and symptoms of inflammation of the lining of the mouth, stomach and/or intestines [mucosal inflammation]).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hycamtin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Keep the blister in the outer carton to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Hycamtin
- The active substance istopotecan. Each capsule contains 0.25 mg or 1 mg of topotecan (as hydrochloride).
- The other ingredients are: hydrogenated vegetable oil, glycerol monostearate, gelatin, titanium dioxide (E171) and only for the 1 mg capsules, red iron oxide (E172). The capsules are printed with black ink containing black iron oxide (E172), shellac, anhydrous ethanol, propylene glycol, isopropanol, butanol, concentrated ammonia solution and potassium hydroxide.
Appearance and packaging
The 0.25 mg Hycamtin capsules are white to off-white and are printed with “Hycamtin” and “0.25 mg”.
The 1 mg Hycamtin capsules are pink and are printed with “Hycamtin” and “1 mg”.
The 0.25 mg and 1 mg Hycamtin capsules are available in packs containing 10 capsules.
Marketing Authorisation Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Farmacéutica S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Novartis Pharmaceuticals UK Limited
Frimley Business Park
Frimley
Camberley, Surrey GU16 7SR
United Kingdom
GlaxoSmithKline Manufacturing S.p.A.
Strada Provinciale Asolana 90
43056 San Polo di Torrile
Parma
Italy
You can request more information about this medicine from the local representative of the Marketing Authorisation Holder:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
България Novartis Bulgaria EOOD Тел: +359 2 489 98 28 | Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Sandoz A/S Tlf: +45 63 95 10 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 0452 555 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Sandoz A/S Tlf: +45 63 95 10 00 |
Ελλάδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España BEXAL FARMACÉUTICA, S.A. Tel: +34 900 456 856 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Sandoz Tél: +33 800 45 57 99 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Sandoz S.R.L. Tel: +40 21 40751 60 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κύπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Sandoz A/S Tel: +45 63 95 10 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 | United Kingdom(Northern Ireland) Novartis Ireland Limited Tel: +44 1276 698370 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site: http://www.ema.europa.eu/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HYCAMTIN 0.25 mg HARD CAPSULESDosage form: CAPSULE, 1 mgActive substance: topotecanManufacturer: Sandoz Pharmaceuticals D.D.Prescription requiredDosage form: INJECTABLE INFUSION, UnknownActive substance: topotecanManufacturer: Sandoz Pharmaceuticals D.D.Prescription requiredDosage form: INJECTABLE PERFUSION, 1 mg/mlActive substance: topotecanManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for HYCAMTIN 0.25 mg HARD CAPSULES
Discuss questions about HYCAMTIN 0.25 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






