HEPCLUDEX 2 mg POWDER FOR INJECTABLE SOLUTION


How to use HEPCLUDEX 2 mg POWDER FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Hepcludex 2 mg powder for solution for injection
bulevirtide
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Hepcludex is and what it is used for
- What you need to know before you use Hepcludex
- How to use Hepcludex
- Possible side effects
- Storage of Hepcludex
- Contents of the pack and other information
- Step-by-step injection guide
1. What Hepcludex is and what it is used for
What Hepcludex is
Hepcludex contains the active substance bulevirtide, which is an antiviral medicine.
What Hepcludex is used for
Hepcludex is used to treat long-term (chronic) infection with the hepatitis delta virus (HDV) in adults with compensated liver disease (when the liver is still working properly). Hepatitis delta virus infection causes liver inflammation.
How Hepcludex works
HDV uses a specific protein from liver cells to enter these cells. Bulevirtide, the active substance in this medicine, blocks the protein and thus prevents HDV from entering liver cells. This reduces the spread of HDV in the liver and reduces inflammation.
2. What you need to know before you use Hepcludex
Do not use Hepcludex:
- if you are allergic to bulevirtide or any of the other ingredients of this medicine (listed in section 6).
If you are unsure, consult your doctor before taking this medicine.
Warnings and precautions
Do not stop treatment with Hepcludex unless your doctor advises you to do so. Stopping treatment may reactivate the infection and worsen your disease.
Consult your doctor or pharmacist before starting treatment with Hepcludex:
- if your liver is not working properly – it is not known how Hepcludex works in these circumstances; if your liver is not working well, it is not recommended to use Hepcludex.
- if you have had kidney disease or if blood tests show kidney problems. Before and during treatment, your doctor may ask for blood tests to check that your kidneys are working properly;
- if you have HIV or hepatitis C infection - it is not known how Hepcludex works in these circumstances; your doctor may ask for blood tests to check the status of your HIV or hepatitis C infection.
Children and adolescents
Children and adolescents under 18 years of age should not be treated with Hepcludex.
Other medicines and Hepcludex
Tell your doctor if you are using, have recently used or might use any other medicines.
Some medicines may increase the side effects of Hepcludex and should not be taken at the same time. This is why you should tell your doctor if you are taking any of these medicines:
- ciclosporin, a medicine that suppresses the immune system;
- ezetimibe, used to treat high blood cholesterol levels;
- irbesartan, used to treat high blood pressure and heart disease;
- ritonavir, used to treat HIV infection;
- sulfasalazine, used to treat rheumatoid arthritis, ulcerative colitis and Crohn's disease.
Some medicines may increase or decrease the effects of Hepcludex when taken together. In some cases, you may need to have some tests or your doctor may need to change the dose or monitor you regularly:
- cancer treatments (e.g. dasatinib, docetaxel, ibrutinib or paclitaxel);
- antihistamines used for allergies (e.g. ebastine or fexofenadine);
- medicines for the immune system (e.g. everolimus, sirolimus or tacrolimus);
- medicines for the treatment of hepatitis C and HIV (e.g. darunavir, glecaprevir, grazoprevir, indinavir, maraviroc, paritaprevir, saquinavir, simeprevir, tipranavir or voxilaprevir);
- medicines for diabetes (e.g. glibenclamide, nateglinide or repaglinide);
- medicines for erectile dysfunction (e.g. avanafil, sildenafil or vardenafil);
- medicines for the treatment of high blood pressure and heart disease (e.g. olmesartan, telmisartan or valsartan);
- statins, medicines used for high blood cholesterol levels (e.g. atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin or simvastatin);
- thyroid hormones used to treat thyroid problems;
- alfentanil, an opioid medicine used to treat severe pain;
- bosentan, used for pulmonary arterial hypertension;
- buspirone, a medicine for anxiety;
- budesonide, used for asthma and chronic obstructive pulmonary disease;
- conivaptan and tolvaptan, used to treat hyponatraemia (low sodium levels);
- darifenacin, used to treat urinary incontinence;
- dronedarone, a medicine for cardiac arrhythmias;
- eletriptan, used for migraine headaches;
- eplerenone, used for hypertension;
- estra-3-sulfate, a hormonal medicine for menopause;
- felodipine and nisoldipine (heart medicines);
- lomitapide, used for high blood cholesterol levels;
- lurasidone and quetiapine, antipsychotic medicines for psychiatric disorders;
- midazolam and triazolam, medicines for insomnia (inability to sleep) and for anaesthesia (to prevent pain during surgery);
- naloxegol, used to treat opioid dependence for severe pain;
- ticagrelor, an anticoagulant to prevent blood clotting.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine. You should not use this medicine unless your doctor specifically advises you to do so.
If you are a woman of childbearing potential, you should not use this medicine without using an effective method of contraception.
Talk to your doctor to decide whether you can breast-feed during treatment with Hepcludex. It is not known whether Hepcludex is excreted in breast milk. Therefore, a decision must be made whether to discontinue breast-feeding or to discontinue Hepcludex treatment.
Driving and using machines
Dizziness and fatigue are side effects that may affect your ability to drive and use machines. If you are unsure, consult your doctor.
Hepcludex contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per ml, which is essentially ‘sodium-free’.
3. How to use Hepcludex
Follow the instructions for administration of this medicine exactly as advised by your doctor. If you are unsure, consult your doctor again.
Dosage
The recommended dose is 2 mg once daily by subcutaneous injection (just under the skin). Your doctor will tell you how long you should use the medicine.
Your doctor and nurse will teach you how to prepare and inject Hepcludex. This leaflet contains a step-by-step injection guide to help you inject the medicine (see section 7).
If you use more Hepcludex than you should
The usual dose is 2 mg (1 vial) per day. If you think you may have received more than you should, tell your doctor immediately.
If you forget to use Hepcludex
If it has been less than 4 hours since you missed a dose of Hepcludex, you should inject the missed dose as soon as possible and administer the next scheduled dose at the usual time.
If it has been more than 4 hours since you missed a dose of Hepcludex, do not inject the missed dose. You should administer the next dose the following day at the usual time. Do not inject a double dose to make up for a missed dose. Inform your doctor if you have missed a dose of Hepcludex.
If you stop treatment with Hepcludex
If you no longer wish to continue using Hepcludex, consult your doctor before stopping treatment. Stopping treatment may reactivate the infection and worsen your disease. Inform your doctor immediately about any change in symptoms after stopping treatment.
If you have any further questions on the use of Hepcludex, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any side effect or if you notice any side effect not mentioned in this leaflet, consult your doctor.
The following side effect is very common(may affect more than 1 in 10 people):
- headache.
The following side effects are common(may affect up to 1 in 10 people):
- dizziness
- nausea
- fatigue
- flu-like illness
- itching
- joint pain
- injection site reactions which may include swelling, redness, irritation, bruising, itching, rash, induration, infection or pain at the injection site.
The following side effects are uncommon(may affect up to 1 in 100 people):
- allergic reactions, including anaphylactic reaction (a life-threatening allergic reaction).
Symptoms of allergic reactions may include:
- shortness of breath or wheezing
- swelling of the face, lips, tongue or throat (angioedema)
- skin rash
- changes in blood pressure or heart rate.
Symptoms of an anaphylactic reaction are the same as those of an allergic reaction, but are more severe and require immediate medical attention.
Blood tests may also show:
- increased bile acids in the blood (very common)
- increased white blood cells (eosinophils) (common).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hepcludex
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and vial after ‘EXP’. The expiry date refers to the last day of that month.
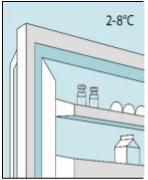
Store in a refrigerator (2°C to 8°C). To protect from light, keep the vials in the outer carton.
The reconstituted solution should be used immediately. However, if this is not possible, it can be stored for a maximum of 2 hours at a temperature of up to 25°C.
Medicines or needles should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines or needles no longer required.
6. Container Contents and Additional Information
Hepcludex Composition
The active ingredient is bulevirtida 2 mg. Each vial contains bulevirtida acetate equivalent to 2 mg of bulevirtida.
The other components are sodium carbonate anhydrous, sodium hydrogen carbonate, mannitol, hydrochloric acid, and sodium hydroxide.
Product Appearance and Container Contents
Bulevirtida is a powder for solution for injection and is presented as a white or off-white powder.
Each box contains 30 individual doses.
Marketing Authorization Holder
Gilead Sciences Ireland UC
Carrigtohill
County Cork, T45 DP77
Ireland
Manufacturer
LYOCONTRACT GmbH
Pulverwiese 1
38871 Ilsenburg
Germany
or
Gilead Sciences Ireland UC
IDA Business and Technology Park
Carrigtohill
Co. Cork
Ireland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Gilead Sciences Belgium SRL-BV Tel: + 32 (0) 24 01 35 50 | Lietuva Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 |
Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | Luxembourg/Luxemburg Gilead Sciences Belgium SRL-BV Tel: + 32 (0) 24 01 35 50 |
Ceská republika Gilead Sciences s.r.o. Tel: + 420 (0) 910 871 986 | Magyarország Gilead Sciences Ireland UC Tel.: + 353 (0) 1 686 1888 |
Danmark Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 | Malta Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Deutschland Gilead Sciences GmbH Tel: + 49 (0) 89 899890-0 | Nederland Gilead Sciences Netherlands B.V. Tel: + 31 (0) 20 718 36 98 |
Eesti Gilead Sciences Poland Sp. z o.o. Tel.: +48 (0) 22 262 8702 | Norge Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 |
Ελλáδα Gilead Sciences Ελλáς Μ.ΕΠΕ. Τηλ: + 30 (0) 210 8930 100 | Österreich Gilead Sciences GesmbH Tel: + 43 (0) 1 260 830 |
España Gilead Sciences, S.L. Tel: + 34 (0) 91 378 98 30 | Polska Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 |
France Gilead Sciences Tél: + 33 (0) 1 46 09 41 00 | Portugal Gilead Sciences, Lda. Tel: + 351 (0) 21 7928790 |
Hrvatska Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | România Gilead Sciences (GSR) S.R.L. Tel: +40 31 631 18 00 |
Ireland Gilead Sciences Ireland UC Tel: + 353 (0) 214 825 999 | Slovenija Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Ísland Gilead Sciences Sweden AB Sími: + 46 (0) 8 5057 1849 | Slovenská republika Gilead Sciences Slovakia s.r.o. Tel: + 421 (0) 232 121 210 |
Italia Gilead Sciences S.r.l. Tel: + 39 02 439201 | Suomi/Finland Gilead Sciences Sweden AB Puh/Tel: + 46 (0) 8 5057 1849 |
Κúπρος Gilead Sciences Ελλáς Μ.ΕΠΕ. Τηλ: + 30 (0) 210 8930 100 | Sverige Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Latvija Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 | United Kingdom (Northern Ireland) Gilead Sciences Ireland UC Tel: + 44 (0) 8000 113 700 |
Date of Last Revision of this Leaflet: <{MM/AAAA}> <{mes AAAA}>.
This medicinal product has been authorized with a "conditional approval". This approval mechanism means that more information on this medicinal product is expected.
The European Medicines Agency will review the new information on this medicinal product at least once a year, and this leaflet will be updated as necessary.
- Step-by-Step Injection Guide
Before using Hepcludex, you must first read sections 1-6 of this leaflet.
Before starting treatment with this medication at home, your doctor or nurse will teach you how to prepare and inject Hepcludex. This guide indicates how you should inject the medication yourself. Consult your doctor or nurse if there is anything you do not understand, if you have any questions, or if you need more information or help. Take the time to prepare and inject Hepcludex carefully.
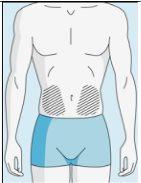
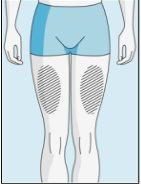
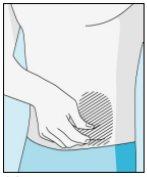
Injection Sites | Abdomen | Upper Thigh | |
To reduce reactions at the injection site, you can change the injection site of bulevirtida regularly. Do not injectbulevirtida into the following areas: knee, groin, lower or inner buttocks, directly into a blood vessel, around the navel, into scar tissue, hematomas, moles, a surgical scar, tattoos, or burns, or where an injection site reaction has occurred. |
|
| |
|
|
|
|
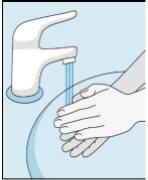
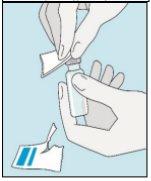
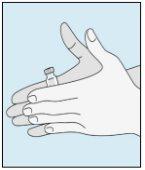
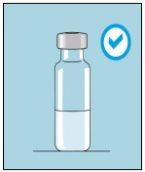
1A Storage | 1B Mixing Dose | 1C Washing Hands | 1D Cleaning the Vial |
The bulevirtida vials should be stored in the original packaging in the refrigerator (between 2 and 8 °C) to protect bulevirtida from light. | Reconstituted bulevirtida should be used immediately. The following instructions are for dissolving a single dose. | Wash your hands thoroughly with soap and warm water and dry them with a clean towel. Once your hands are clean, do not touch anything other than the medication, auxiliary materials, and the area surrounding the injection site. | Rub the top of the vial with a new cotton ball soaked in alcohol and let it air dry. If you touch the rubber stopper after cleaning, clean it again with another cotton ball soaked in alcohol. |
|
|
| |
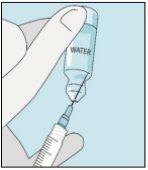
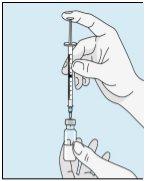
2A Withdrawing Sterile Water | 2B Injecting Sterile Water into the Powder | 2C Gently Mixing Bulevirtida | |
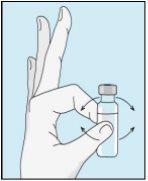
Take the syringe. Place the longest needle on it. Important!Make sure the capped needle is securely attached by gently pressing and twisting it clockwise. Remove the plastic cap. Open the sterile water for injectable preparations. Insert the needle into the vial and gently invert the water vial. Make sure the needle tip is always below the surface of the water to prevent air bubbles from entering the syringe. Slowly pull the plunger until you have 1.0 cc/ml of sterile water inside the syringe. Carefully remove the needle and syringe from the vial. | Gently tap the bulevirtida vial to loosen the powder. Insert the needle with sterile water into the bulevirtida vial at an angle. Slowly inject the sterile water so that it trickles down the side of the vial onto the bulevirtida powder. | Gently tap the bulevirtida vial with your fingertips for 10 seconds to start dissolving the powder. Then, gently rotate the bulevirtida vial between your hands to mix it completely. Make sure there is no bulevirtida powder stuck to the sides of the vial. Important!Do not shake the bulevirtida vial. If you shake it, foam will form, and the medication will take much longer to dissolve. | |
|
| ||
2D Inspecting Bulevirtida | 2E Bulevirtida Ready for Injection | ||
Once the powder starts to dissolve, let it sit, and it will completely dissolve. After tapping, it may take up to 3 minutes to dissolve. | When it is fully mixed, the bulevirtida solution should be clear. Important!Fully dissolved bulevirtida should be clear and free of foam. If the bulevirtida solution is foamy or yellowish, let it dissolve for a longer time. If you see bubbles, gently tap the vial until they disappear. If you notice particles in the bulevirtida solution once it is fully dissolved, do not use that vial. Consult the doctor or pharmacist who supplied it to you. |
|
|
|
|
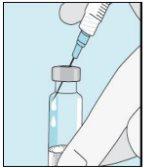
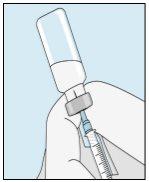
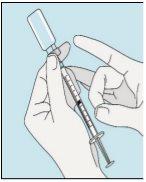
3A Inserting the Needle into the Vial | 3B Withdrawing Bulevirtida | 3C Completing Preparation | 3D Changing and Discarding the Needle |
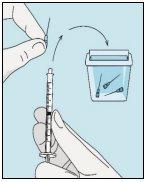
Take the syringe. Insert the needle into the bulevirtida liquid vial. | Gently invert the vial. Make sure the needle tip is always below the surface of the bulevirtida solution so that no air bubbles enter the syringe. Slowly pull the plunger to draw 1.0 cc/ml of bulevirtida into the syringe. | Gently tap or shake the syringe and push/pull the plunger to eliminate any additional air and bubbles. To ensure you end up with 1.0 cc/ml of bulevirtida in the syringe, you may need to pull the plunger past the 1.0 cc/ml mark. Carefully remove the needle and syringe from the vial. | Remove the longer needle from the syringe and discard it properly so that no one can get hurt. Important!Do not put the plastic cap back on the needle. |
|
|
|
|
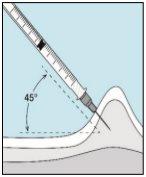
3E Inserting the Needle for Injection | 3F Choosing the Injection Site | 3G Preparing the Injection Site | 3H Injecting Bulevirtida |
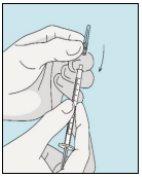
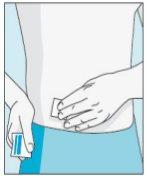
Place the shorter needle on the syringe. Important!Make sure the capped needle is securely attached by gently pressing and twisting it clockwise. Remove the plastic cap. | Choose a different site from the one you used for your last injection. Clean the injection site with a new cotton ball soaked in alcohol. Start in the center, apply pressure, and clean in a circular motion outward. Important!Let the area air dry. Prepare the bulevirtida vial. Clean the top of the bulevirtida vial again with a new cotton ball soaked in alcohol. Let it air dry. | Pinch the skin around the injection site. | Puncture the skin at a 45-degree angle. Most of the needle should be inserted. Slowly push the plunger all the way to inject bulevirtida. Remove the needle from the skin. Remove the needle from the syringe and discard both properly so that no one can get hurt (see 3D). |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HEPCLUDEX 2 mg POWDER FOR INJECTABLE SOLUTIONDosage form: TABLET, 150 mgActive substance: maravirocManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: TABLET, 300 mgActive substance: maravirocManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: INJECTABLE, 90 mgActive substance: enfuvirtideManufacturer: Roche Registration GmbhPrescription required
Online doctors for HEPCLUDEX 2 mg POWDER FOR INJECTABLE SOLUTION
Discuss questions about HEPCLUDEX 2 mg POWDER FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions