FUZEON 90 mg/ml POWDER AND SOLVENT FOR INJECTABLE SOLUTION


How to use FUZEON 90 mg/ml POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Fuzeon 90mg/ml powder and solvent for solution for injection
Enfuvirtide
Read allof this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Fuzeon and what is it used for
- What you need to know before you use Fuzeon
- How to use Fuzeon
- Possible side effects
- Storing Fuzeon
- Contents of the pack and further information
- Step-by-step guide on how to inject Fuzeon
1. What is Fuzeon and what is it used for
What is Fuzeon
Fuzeon contains the active substance “enfuvirtide” and belongs to a group of medicines called “antiretrovirals”.
What Fuzeon is used for
Fuzeon is used for the treatment of the Human Immunodeficiency Virus (HIV) in combination with other antiretroviral medicines in patients infected with HIV.
- Your doctor has prescribed Fuzeon to help control your HIV infection.
- Fuzeon does not cure HIV infection.
How Fuzeon works
HIV attacks cells in your blood known as CD4 or T lymphocytes. The virus needs to come into contact with them and gets inside these cells to multiply. Fuzeon helps prevent this.
2. What you need to know before you use Fuzeon
Do not use Fuzeon if
- you are allergic to enfuvirtide or any of the other ingredients of this medicine (listed in section 6).
If you are not sure, talk to your doctor, pharmacist, or nurse before using Fuzeon.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting Fuzeon if:
- you have ever had any lung problems
- if you have ever had any kidney problems.
- if you have chronic hepatitis B or C or other liver disease – you are more likely to have serious liver problems while taking this medicine
Signs of previous infections
In some patients with advanced HIV infection (AIDS) and a history of opportunistic infections, signs and symptoms of inflammation of previous infections may appear soon after starting anti-HIV treatment. These symptoms are thought to be due to an improvement in the body's immune response. This improvement enables the body to fight infections that were already present but not showing any symptoms. If you notice any symptoms of infection, please inform your doctor immediately.
Signs of autoimmune disorders
In addition to opportunistic infections, autoimmune disorders (a condition that occurs when the immune system attacks healthy body tissue) may also appear after you have started taking medicines for the treatment of your HIV infection. Autoimmune disorders may appear many months after starting treatment. If you notice any symptoms of infection or other symptoms such as muscle weakness, weakness starting in the hands and feet and moving up towards the trunk of the body, palpitations, tremors, or hyperactivity, inform your doctor immediately to receive the necessary treatment.
Patients with liver disease
Patients with chronic hepatitis B or C who are on anti-HIV therapy are at a greater risk of experiencing serious liver problems. Talk to your doctor if you have a history of liver disease.
Bone disease (osteonecrosis)
Some patients who receive combination anti-HIV therapy may develop a bone disease called osteonecrosis. Bone tissue dies because the blood supply to the bone is lost (bone tissue death due to loss of blood supply to the bone).
- Signs of osteonecrosis are stiffness in the joints, pain, and discomfort (especially in the hips, knees, and shoulders) and difficulty moving. If you notice any of these symptoms, inform your doctor.
- Risk factors for developing this disease include: how long you have been taking anti-HIV medicines, if you take corticosteroids, how much alcohol you drink, how well your immune system is working, and being overweight.
Using Fuzeon with other medicines
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines. This includes medicines without a prescription and herbal medicines. Fuzeon has not shown interactions with other medicines that are part of your anti-HIV treatment or with rifampicin (an antibiotic).
Using Fuzeon with food and drinks
You can use Fuzeon with or without food. However, you should follow the instructions indicated in the leaflets of the other medicines you are taking.
Pregnancy and breastfeeding
- If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. You should not use Fuzeon unless your doctor specifically tells you to.
- It is recommended that HIV-infected women do not breastfeed their babies because HIV infection can be passed on to the baby through breast milk. If you are breastfeeding or thinking of breastfeeding, talk to your doctor as soon as possible.
Driving and using machines
The effects of Fuzeon on the ability to drive or use machines have not been studied. You should not drive or use machines if you feel dizzy while using Fuzeon.
Fuzeon contains sodium
Fuzeon contains less than 1 mmol of sodium (23 mg) per dose; this is essentially “sodium-free”.
3. How to use Fuzeon
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
How to prepare and inject Fuzeon
Fuzeon must be administered as an injection under the skin – called “subcutaneous injection”. In section 7, it is indicated how to prepare Fuzeon and how to self-administer an injection.
How much to use
- The recommended dose is 90 mg, twice a day, for adults and adolescents (16 years and older).
- It is administered as a 1 ml injection under the skin.
- It is best to use Fuzeon at the same time every day.
- Try to space the doses conveniently apart at times that are good for you – for example, in the morning and in the afternoon.
Consult the end of this leaflet for additional information on how to use Fuzeon (see section 7). There you will find instructions on how to prepare Fuzeon and how to administer the injection yourself.
If you use more Fuzeon than you should
If you use more Fuzeon than you should, talk to your doctor or go to the hospital immediately. Take the medicine package with you.
If you forget to use Fuzeon
- If you forget to use a dose, inject the dose as soon as you remember. However, if there are less than 6 hours before your next scheduled dose, do not take the missed dose.
- Do not inject a double dose to make up for the missed dose.
If you stop using Fuzeon
- Continue using your medicine until your doctor tells you to stop. If you stop and there is an interruption in your treatment, it may speed up the possibility that the HIV in your blood becomes resistant to Fuzeon. This is less likely if you use it regularly and without interruptions in your treatment.
- Over time, the HIV virus in your blood may become resistant to Fuzeon. If this happens, your viral load will start to increase. Your doctor may decide not to continue treating you with Fuzeon. Your doctor will discuss this with you at that time.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Fuzeon and contact your doctor immediately if you notice any of the following serious side effects because you may need urgent medical treatment:
- Allergic reaction (hypersensitivity) – the signs may include: rash, high temperature or chills, feeling tired or unwell, sweating or shivering.
This side effect is rare (affects less than 1 in 1,000 people). These signs do not necessarily mean that you are allergic to this medicine.
Talk to your doctor if you have side effects at the injection site
The most common side effects (affect more than 1 in 10 people) are problems at the injection site. It is very likely that you will have one or more of the following reactions, which are usually mild to moderate:
- Redness
- Swelling
- Itching
- Bruising
- Hardening of the skin or lumps
- Pain, feeling of pain, or discomfort
These reactions may appear in the first week of treatment and usually only last up to 7 days. They usually do not get worse after this time. If you have any of these reactions, do not stop using Fuzeon but talk to your doctor.
These reactions may worsen when the injection is repeated at the same site on the body. They may also worsen when the injection is given more deeply than intended (for example, into the muscle). Rarely, you may have an infection at the injection site. To minimize the risk of infection, it is important that you follow the instructions in section 7.
Fuzeon may cause a buildup of a type of protein called amyloid under the skin at the injection site. You may notice lumps under the skin. Please contact your doctor if this happens.
Other possible side effects
Very common(affect more than 1 in 10 people)
- Diarrhea
- Feeling unwell
- Weight loss
- Pain and feeling of numbness in hands, feet, or legs.
Common(affect up to 1 in 10 people)
- Pneumonia
- Ear infection
- Enlarged lymph nodes (lymph node swelling)
- Inflamed eyes (conjunctivitis)
- Flu or flu-like symptoms
- Inflamed breasts
- Nasal congestion
- Anorexia
- Acid reflux
- Pancreatitis
- Loss of appetite
- Diabetes
- Nightmares
- Feeling dizzy
- Shakiness (tremor)
- Feeling anxious or irritable
- Being unable to concentrate
- Decreased sensitivity
- Acne
- Redness of the skin
- Eczema
- Dry skin
- Warts
- Muscle pain
- Kidney stones
- Feeling weak
- Blood in the urine
- Changes shown in blood tests (increased fat in the blood)
Reporting side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Fuzeon
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the Fuzeon vials or on the label of the vials of Water for Injections after “EXP”. The expiry date is the last day of the month shown.
Store the vial in the outer packaging to protect it from light.
Once the solution is prepared, the injection should be administered immediately. If it is not administered immediately, store it in the refrigerator (between 2°C and 8°C) and use it before 24 hours.
Do not use this medicine if you notice any particles in the powder or in the solution after adding the water for injections. Also, do not use the water for injections if you notice particles in the vial or if the water is cloudy.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Fuzeon
- The active ingredient is enfuvirtide. Each vial contains 108 mg of enfuvirtide. After reconstitution with the solvent included in the package, 1 ml of reconstituted solution contains 90 mg of enfuvirtide.
- The other components are:
Powder
Sodium carbonate anhydrous
Mannitol
Sodium hydroxide
Hydrochloric acid
Solvent
Water for injectable preparations
See section 2 "Fuzeon contains sodium".
Appearance of the Product and Container Contents
Fuzeon, powder and solvent for injectable solution, consists of a package containing:
60 vials of Fuzeon
60 vials of Water for injectable preparations used to reconstitute the Fuzeon powder
60 3-ml syringes
60 1-ml syringes
180 alcohol swabs
This package includes everything needed to prepare and inject Fuzeon for 30 days of treatment.
Marketing Authorization Holder
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
The manufacturer responsible for batch release is
Roche Pharma AG
Emil-Barell-Str. 1,
D-79639 Grenzach-Wyhlen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
|
Czech Republic Roche s.r.o. Tel: +420 - 2 20382111 | Malta (See Ireland) |
Denmark Roche Pharmaceuticals A/S Tlf: +45 - 36 39 99 99 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Germany Roche Pharma AG Tel.: +49 (0) 7624 140 | Norway Roche Norge AS Tel.: +47 - 22 78 90 00 |
Estonia Roche Eesti OÜ Tel.: + 372 - 6 177 380 | Austria Roche Austria GmbH Tel.: +43 (0) 1 27739 |
Greece Roche (Hellas) A.E. Tel.: +30 210 61 66 100 | Poland Roche Polska Sp.z o.o. Tel.: +48 - 22 345 18 88 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Portugal Roche Farmacêutica Química, Lda Tel.: +351 - 21 425 70 00 |
France Roche Tel: +33 (0) 1 47 61 40 00 | Romania Roche România S.R.L. Tel.: +40 21 206 47 01 |
Croatia Roche d.o.o. Tel.: +385 1 4722 333 | Slovenia Roche farmacevtska družba d.o.o. Tel.: +386 - 1 360 26 00 |
Ireland Roche Products (Ireland) Ltd. Tel.: +353 (0) 1 469 0700 | Slovak Republic Roche Slovensko, s.r.o. Tel.: +421 - 2 52638201 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Tel: +354 540 8000 | Finland Roche Oy Tel: +358 (0) 10 554 500 |
Italy Roche S.p.A. Tel.: +39 - 039 2471 | Sweden Roche AB Tel.: +46 (0) 8 726 1200 |
Cyprus Γ.Α.Σταμ?της & Σια Λτδ. Tel.: +357 - 22 76 62 76 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Latvia Roche Latvija SIA Tel.: +371 - 6 7039831 | |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/.
- STEP-BY-STEP GUIDE ON HOW TO INJECT FUZEON
Follow exactly the administration instructions of this medication indicated by your doctor or pharmacist. In case of doubt, consult your doctor, pharmacist, or nurse again.
What to Do if You Are Left-Handed
The drawings in this leaflet show right-handed people. If you are left-handed, act naturally. You may find it more convenient:
? to hold the syringe with your left hand and
? to take the vial between the thumb and index finger of your right hand.
When to Ask for Help from Support Staff
At first, it may be difficult to inject in some places, such as the arm. Ask for help if you need it, to your partner, a friend, or a family member. You may want to ask someone to accompany you to a training session on the injection technique with your doctor or nurse.
The Syringes
The syringes supplied with this medication have a colored needle protector. This is attached to the needle and covers the needle after use to reduce the risk of accidentally pricking someone else with the needle. Although these syringes have this safety feature, it is important that you dispose of them properly after use. Follow the instructions given by your doctor, pharmacist, or nurse.
Safety Tips
? Wash your hands thoroughly. This will reduce the risk of bacterial infections.
? Once you have washed your hands, do not touch anything except the medication and the material provided for the injection.
? When handling the syringe, do not touch the needle.
? Do not touch the vial stoppers once they have been cleaned with the alcohol swabs.
? Never use opened materials. Before using, check that all elements of the package are closed.
? Never use or share used needles.
? Never use a bent or damaged needle.
? Never mix the medication with tap water.
? Never inject the medication with other injectable medications.
? Only inject Fuzeon under the skin ("subcutaneously").
? Do not inject Fuzeon into a vein ("intravenously") or into a muscle ("intramuscularly").
? Dispose of all used material in the container with a lid for disposable materials. Do this even if the vials contain unused amounts of medication or water for injectable preparations, as these are for single use. Consult your doctor, pharmacist, or nurse if you have any doubts about the safe disposal of this material.
The following is a basic, step-by-step guide to injecting the medication.
Step A: To Begin
- Gather the following materials:
? A vial of Fuzeon (glass container with white powder inside)
? A vial of water for injectable preparations (glass container with clear, colorless liquid inside)
? A 3-ml syringe (large syringe) with a 25-mm needle
? A 1-ml syringe (small syringe) with a 13-mm needle
? Three alcohol swabs
? A container with a lid for safe disposal of disposable materials
- Open the syringe packages and remove the vial caps.
? Place the packages and vial caps in the container with a lid for disposable materials.
? Place the syringes and vials on a clean surface.
- Wash your hands thoroughly.
? After washing your hands, do not touch anything except the injection material and the area where you will administer the injection.
- Clean the vial stoppers.
? Clean each vial stopper with a new, clean alcohol swab. Let the stopper air dry.
? Make sure you do not touch the rubber stoppers once they are cleaned. If you do, make sure to clean them again.
Step B: Preparation of the Fuzeon Mixture
Withdraw Water for Injectable Preparations
- Take the large 3-ml syringe. Use your index finger to move the colored needle protector away from the needle.
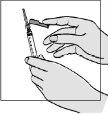
- To ensure the needle is firmly attached to the syringe:
? hold the plastic cap below the needle protector
? gently twist and push the needle and cap into the syringe. Do not apply too much force, as the needle may loosen.
- To remove the transparent plastic cap:
? hold the syringe and pull off the cap.
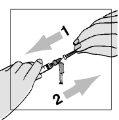
- Aspirate 1.1 ml of air
- Insert the syringe needle into the rubber stopper of the vial with the water for injectable preparations and press the plunger. This injects the air.
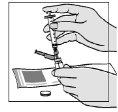
- Gently invert the vial. Make sure the needle tip remains below the surface of the water for injectable preparations so that no air bubbles enter the syringe.
- Slowly withdraw the water by pulling the plunger until the 1.1-ml mark. Please note that the vial contains more liquid than you need (2 ml); you only need to withdraw 1.1 ml to prepare your injection properly.
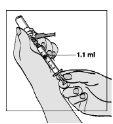
- Gently tap the syringe to make any air bubbles rise.
? If too much air has entered the syringe, gently push the plunger to return the air bubbles to the vial.
? Then withdraw the water again,
? Make sure you have 1.1 ml of water for injectable preparations in the syringe.
? This step can be repeated until you have the correct amount of water for injectable preparations in the syringe.
- Remove the needle from the vial. Make sure you do not touch the needle with your fingers or anything else at any time.
- Discard the vial and the water for injectable preparations in the container with a lid for disposable materials - this vial is for single use.
Inject Water for Injectable Preparations into the Fuzeon Vial
- Gently tap the vial to distribute the powder.
- Hold the main part of the syringe containing the water and insert the needle through the rubber stopper of the vial with a slight inclination.
- Slowly press the syringe plunger.
? Allow the water to flow down the inner walls of the vial.
? Avoid injecting the water abruptly onto the powder, as foam may form.
? If foam forms, the powder may take longer to dissolve completely.
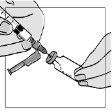
- Once you have injected all the water for injectable preparations into the Fuzeon vial, remove the syringe from the vial.
- Hold the syringe by the main part with one hand and, on a flat surface, gently press down the colored needle protector until the needle is covered by the protector.
- You will hear a click. Do not use your free hand to press the protector onto the needle.
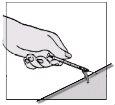
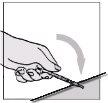
- Discard the syringe in a container with a lid for disposable materials.
Mixing Water for Injectable Preparations with Fuzeon Powder
- Gently tap the vial with your fingertips until the powder starts to dissolve. Never shake the vial or turn it upside down to mix, as a lot of foam may form.
- When the powder starts to dissolve, set the vial aside and let it dissolve completely.
? The powder may take up to 45 minutes to dissolve.
? The patient can gently roll the vial between their hands after adding the water for injectable preparations until the powder is completely dissolved.
? This can reduce the time it takes to dissolve.
- After the powder has dissolved completely
? Let any bubbles that have formed settle.
? If there are still bubbles, gently tap the sides of the vial to make them settle.
- It is essential to check if the liquid contains particles (fragments).
? If you notice any particles in the liquid, do not use it.
? Discard the vial in the container with a lid for disposable materials or return it to the pharmacy. Start again with a new vial of Fuzeon powder.
- If you accidentally touch the rubber stopper, make sure to clean it again with a new alcohol swab.
- Once the dose is mixed with the water for injectable preparations, it must be used immediately. If not used, it can be stored in the refrigerator and used before 24 hours.
? Wait for the liquid to reach room temperature before using it.
- If you are preparing the two daily doses at the same time, make sure to use new syringes, new water for injectable preparations, and a new vial of Fuzeon for each dose.
Step C: Preparation for Administration of the Injection
Withdraw Fuzeon with the 1-ml Syringe
- Clean the Fuzeon vial stopper again with a new alcohol swab.
- Take the small 1-ml syringe. Use your index finger to move the colored needle protector away from the needle.
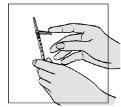
- To ensure the needle is firmly attached to the syringe:
? hold the plastic cap below the needle protector
? gently twist and push the needle and cap into the syringe.
- To remove the transparent plastic cap:
? hold the syringe and pull off the cap.
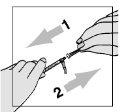
- Aspirate 1 ml of air.
? Be careful not to pull the plunger too quickly - you might exceed the 1-ml mark or pull out the syringe.
- Insert the syringe needle into the rubber stopper of the Fuzeon vial and press the plunger. This injects the air.
- Gently invert the vial several times.
Make sure the needle tip remains below the surface of the solution so that no air bubbles enter the syringe.
- Slowly pull the plunger until the solution reaches the 1.0-ml mark.
? Be careful not to pull the plunger too quickly - you might exceed the 1-ml mark or pull out the syringe.
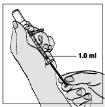
- Gently tap the syringe to make any air bubbles rise.
? If too much air enters the syringe, gently push the plunger to return the air to the vial.
? Then withdraw the liquid again.
? Make sure you have 1.0 ml of liquid in the syringe (or the amount prescribed by your doctor, if different).
? This step can be repeated until the correct amount of solution is inside the syringe.
- Remove the syringe from the vial.
Step D: Injection of Fuzeon
Advice:Your doctor or nurse will suggest different injection techniques that may be more suitable for you.
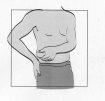
Where to Inject



? Fuzeon is administered in a 1-ml injection under the skin - known as "subcutaneous injection".
? You can inject it into your arms, the front of your thigh, or the abdominal area (abdomen).
? Choose a different area from the last injection you received.
? Do not inject the medication into a site where there is still a reaction from the previous dose. Check the areas where there may be a reaction by pressing the skin to see if there are hard lumps.
? Do not inject the medication into areas that may be irritated by a belt or by rubbing against clothing.
? Do not inject the medication into moles, scars, bruises, or the navel.
Clean the Injection Site
Clean the injection site with an alcohol swab in a circular motion outward. Let the area dry completely.
Insert the Needle and Administer the Injection
- Pinch the skin to form a fold as large as possible - without hurting yourself.
- Insert the needle into the skin at a 45-degree angle.
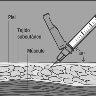
- When you insert the needle:
? release the skin
? with your free hand, hold the main part of the syringe - this will help keep it straight and prevent it from moving.
- With the thumb of your other hand, push the plunger to inject the liquid.
- Hold the main part of the syringe with one hand
- Discard the syringe in a container with a lid for disposable materials.
- If there is any blood where the injection was given, cover the skin with a bandage.
Once the entire dose has been injected, remove the needle from the skin.
After removing the needle
Then, over a flat surface, gently press down on the colored needle shield until the needle is covered by the shield.
You will hear a click.
Do not use your free hand to press the shield over the needle.
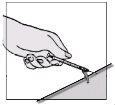
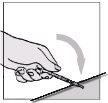
Step E: How to dispose of used materials
Discard all used materials directly into the container with a lid for disposable materials. Do this even if the vials contain remnants of the medication or water for injectable preparations, as they are for single use.
Always keep the container lid closed and place it out of the reach of children.
Ask your doctor, pharmacist, or nurse how to properly dispose of the container.
If you have any doubts or concerns about the safe disposal of materials, consult with your doctor, pharmacist, or nurse.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FUZEON 90 mg/ml POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: TABLET, 150 mgActive substance: maravirocManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: TABLET, 300 mgActive substance: maravirocManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: INJECTABLE, 2 mgActive substance: bulevirtideManufacturer: Gilead Sciences Ireland Unlimited CompanyPrescription required
Online doctors for FUZEON 90 mg/ml POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about FUZEON 90 mg/ml POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions