GONAL-F 900 IU/1.44 ML PRE-FILLED PEN INJECTION SOLUTION

How to use GONAL-F 900 IU/1.44 ML PRE-FILLED PEN INJECTION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
GONAL-f 900 UI/1.44 ml solution for injection in a pre-filled pen
follicle-stimulating hormone alpha
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What GONAL-f is and what it is used for
- What you need to know before you use GONAL-f
- How to use GONAL-f
- Possible side effects
- Storing GONAL-f
- Contents of the pack and other information
Instructions for use
1. What GONAL-f is and what it is used for
What GONAL-f is
GONAL-f contains a substance called ‘follicle-stimulating hormone alpha’. This is a type of ‘follicle-stimulating hormone’ (FSH), which belongs to the family of hormones known as ‘gonadotropins’. Gonadotropins are involved in reproduction and fertility.
What GONAL-f is used for
In adult women,GONAL-f is used:
- to help release an egg from the ovary (ovulation) in women who cannot ovulate and who have not responded to treatment with a substance called ‘clomiphene citrate’.
- in combination with another substance called ‘lutropin alpha’ (‘luteinizing hormone’ or LH), to help release an egg from the ovary (ovulation) in women whose body produces very small amounts of gonadotropins (FSH and LH).
- to help develop several follicles (each containing an egg) in women undergoing assisted reproduction techniques (techniques that can help you become pregnant), such as ‘in vitro fertilization’, ‘intratubal gamete transfer’, or ‘intratubal embryo transfer’.
In adult men,GONAL-f is used:
- in combination with another substance called ‘human chorionic gonadotropin’ (hCG), to help produce sperm in men who are infertile due to low levels of certain hormones.
2. What you need to know before you use GONAL-f
Before starting treatment, your fertility and that of your partner should be assessed by a doctor experienced in the treatment of fertility disorders.
Do not use GONAL-f
- if you are allergic to follicle-stimulating hormone or any of the other ingredients of this medicine (listed in section 6).
- if you have a tumor in the hypothalamus or pituitary gland (both are parts of the brain).
- if you are a woman:
- with enlarged ovaries or fluid-filled sacs in the ovaries (ovarian cysts) of unknown origin.
- with unexplained vaginal bleeding.
- with cancer of the ovaries, uterus, or breast.
- if you have a condition that normally makes pregnancy impossible, such as premature ovarian failure (early menopause) or a malformation of the reproductive organs.
- if you are a man:
- with damaged testes that cannot be cured.
Do not use GONAL-f if any of the above conditions apply to you. If you are not sure, consult your doctor before starting treatment with this medicine.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting treatment with GONAL-f.
Porphyria
Tell your doctor before starting treatment if you or a family member has porphyria (a disorder that can be passed from parents to children).
Inform your doctor immediately if:
- your skin becomes fragile and blisters easily, especially in areas frequently exposed to the sun, and/or
- you experience stomach, arm, or leg pain.
In these cases, your doctor may recommend that you interrupt treatment.
Ovarian Hyperstimulation Syndrome (OHSS)
If you are a woman, this medicine increases the risk of OHSS. This occurs when your follicles develop too much and become large cysts. If you experience pelvic pain, rapid weight gain, nausea, vomiting, or difficulty breathing, consult your doctor immediately, who may interrupt treatment (see section 4).
In case you do not ovulate and you follow the recommended dose and dosing schedule, this syndrome is less likely to occur. Treatment with GONAL-f rarely causes a severe ovarian hyperstimulation syndrome, unless the medicine used for final follicular maturation (containing human chorionic gonadotropin, hCG) is administered. In case of OHSS, your doctor may not prescribe hCG in this treatment cycle and advise you to abstain from intercourse or use barrier contraceptives for at least 4 days.
Multiple Pregnancy
If you use GONAL-f, you have a higher risk of becoming pregnant with more than one baby at the same time (‘multiple pregnancy’, usually twins), than if you become pregnant through natural conception. Multiple pregnancy can cause medical complications for you and your babies. You can reduce the risk of multiple pregnancy by using the correct dose of GONAL-f at the right times. If you undergo assisted reproduction techniques, the risk of multiple pregnancy is related to your age and the quality and number of eggs fertilized or embryos placed inside you.
Miscarriage
If you undergo assisted reproduction techniques or ovarian stimulation to produce eggs, the likelihood of having a miscarriage is higher than in the average population of women.
Blood Clotting Problems (Thromboembolic Events)
If you or a family member has recently or in the past suffered from blood clots in the leg or lung, heart attack, or stroke, you may have a higher risk of experiencing these problems or worsening them with treatment with GONAL-f.
Men with High FSH Levels in the Blood
If you are a man, very high FSH levels in the blood can be a sign of testicular damage. GONAL-f is usually not effective in these cases.
If your doctor decides to try treatment with GONAL-f, to monitor the treatment, your doctor may ask you to have a semen analysis 4 to 6 months after starting treatment.
Children and Adolescents
GONAL-f should not be used in children and adolescents under 18 years of age.
Other Medicines and GONAL-f
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
- If you use GONAL-f with other medicines that help ovulation (e.g., hCG or clomiphene citrate), the response of your follicles may be increased.
- If you use GONAL-f at the same time as an agonist or antagonist of ‘gonadotropin-releasing hormone’ (GnRH) (these medicines decrease the levels of sex hormones and stop ovulation), you may need a higher dose of GONAL-f to produce follicles.
Pregnancy and Breast-feeding
Do not use GONAL-f if you are pregnant or breast-feeding.
Driving and Using Machines
This medicine is not expected to affect your ability to drive or use machines.
GONAL-f Contains Sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially ‘sodium-free’.
3. How to Use GONAL-f
Follow the instructions for administration of this medicine exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist again.
Using this Medicine
- GONAL-f is designed to be administered by injection just under the skin (subcutaneously). The pre-filled pen can be used for several injections.
- The first injection of GONAL-f should be administered under the supervision of your doctor.
- Your doctor or nurse will teach you how to use the GONAL-f pre-filled pen to inject the medicine.
- If you are self-administering GONAL-f, read and follow the “Instructions for Use” carefully.
How Much to Use
Your doctor will decide how much medicine to administer and how often. The doses described below are expressed in International Units (IU).
Women
If you are not ovulating and have irregular periods or no periods.
- GONAL-f is usually administered every day.
- If you have irregular periods, start using GONAL-f on the first 7 days of your menstrual cycle. If you do not have periods, you can start using the medicine on any day that is convenient for you.
- The initial dose of GONAL-f is usually individualized and may be adjusted stepwise.
- The daily dose of GONAL-f should not exceed 225 IU.
- When the desired response is obtained, you will be given a single injection of 250 micrograms of ‘recombinant human chorionic gonadotropin’ (r-hCG, a hCG produced in a laboratory using a special DNA technique), or 5,000 to 10,000 IU of hCG, 24 to 48 hours after the last injection of GONAL-f. The best time to have intercourse is on the same day as the hCG injection and the day after.
If your doctor does not observe the desired response, the continuation of that treatment cycle with GONAL-f should be evaluated and managed according to usual clinical practice.
If an excessive response is obtained, treatment will be interrupted and hCG will not be administered (see section 2, “Ovarian Hyperstimulation Syndrome (OHSS)”). For the next cycle, your doctor will administer a lower dose of GONAL-f than in the previous cycle.
If you have been diagnosed with very low levels of FSH and LH
- The usual initial dose of GONAL-f is 75 to 150 IU, in combination with 75 IU of lutropin alpha.
- You will use these two medicines every day for up to 5 weeks.
- The dose of GONAL-f may be increased every 7 or 14 days, in steps of 37.5 to 75 IU, until the desired response is obtained.
- When the desired response is obtained, you will be given a single injection of 250 micrograms of ‘recombinant human chorionic gonadotropin’ (r-hCG, a hCG produced in a laboratory using a special DNA technique), or 5,000 to 10,000 IU of hCG, 24 to 48 hours after the last injection of GONAL-f and lutropin alpha. The best time to have intercourse is on the same day as the hCG injection and the day after. Alternatively, intrauterine insemination or another medically assisted reproduction procedure may be performed, at the discretion of your doctor.
If your doctor does not observe the desired response after 5 weeks, that treatment cycle with GONAL-f should be interrupted. For the next cycle, your doctor will administer a higher initial dose of GONAL-f than in the canceled cycle.
If an excessive response is obtained, treatment will be interrupted and hCG will not be administered (see section 2, “Ovarian Hyperstimulation Syndrome (OHSS)”). For the next cycle, your doctor will administer a lower dose of GONAL-f than in the previous cycle.
If you need to develop several eggs before any assisted reproduction technique
- The initial dose of GONAL-f is usually individualized and may be adjusted stepwise up to a maximum of 450 IU per day.
- Treatment continues until the eggs have developed to the desired point. Your doctor will check this through blood tests and/or ultrasound scans.
- When the eggs are ready, you will be given a single injection of 250 micrograms of ‘recombinant human chorionic gonadotropin’ (r-hCG, a hCG produced in a laboratory using a special DNA technique), or 5,000 to 10,000 IU of hCG, 24 to 48 hours after the last injection of GONAL-f. This makes your eggs ready for retrieval.
Men
- The usual dose of GONAL-f is 150 IU, in combination with hCG.
- You will use these two medicines three times a week, for at least 4 months.
- If you have not responded to treatment after 4 months, your doctor may suggest that you continue using these two medicines for at least 18 months.
If you use more GONAL-f than you should
The effects of using too much GONAL-f are not known. However, it can be expected that ovarian hyperstimulation syndrome (OHSS) may occur, which is described in section 4. However, this syndrome will only occur if hCG is also administered (see section 2, “Ovarian Hyperstimulation Syndrome (OHSS)”).
If you forget to use GONAL-f
If you forget to use GONAL-f, do not take a double dose to make up for forgotten doses. Consult your doctor as soon as you realize you have forgotten a dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious Side Effects in Women
- Pelvic pain, accompanied by nausea or vomiting, can be symptoms of ovarian hyperstimulation syndrome (OHSS). This can indicate that your ovaries have overreacted to treatment and developed large cysts (see section 2, “Ovarian Hyperstimulation Syndrome (OHSS)”). This side effect is common (affects up to 1 in 10 people).
- Ovarian hyperstimulation syndrome can worsen with clearly enlarged ovaries, decreased urine production, weight gain, difficulty breathing, and/or possible fluid accumulation in the abdomen or chest. This side effect is uncommon (affects up to 1 in 100 people).
- In rare cases, complications of ovarian hyperstimulation syndrome such as ovarian torsion or blood clotting can occur (affects up to 1 in 1,000 people).
- In very rare cases (affects up to 1 in 10,000 people), serious complications of blood clotting (thromboembolic events) can occur, sometimes independent of ovarian hyperstimulation syndrome. This could cause chest pain, shortness of breath, stroke, or heart attack (see also section 2, “Blood Clotting Problems”).
Serious Side Effects in Men and Women
- Allergic reactions, such as skin rash, redness of the skin, blisters, swelling of the face with difficulty breathing, can sometimes be severe. This side effect is very rare (affects up to 1 in 10,000 people).
If you experience any of the above side effects, you should immediately consult your doctor, who may ask you to interrupt treatment with GONAL-f.
Other Side Effects in Women
Very common (affects more than 1 in 10 people):
- Fluid-filled sacs in the ovaries (ovarian cysts).
- Headache.
- Local reactions at the injection site, such as pain, redness, bruising, swelling, and/or irritation.
Common (affects up to 1 in 10 people):
- Abdominal pain.
- Nausea, vomiting, diarrhea, abdominal cramps, and flatulence.
Very rare (affects up to 1 in 10,000 people):
- Allergic reactions, such as skin rash, redness of the skin, blisters, swelling of the face with difficulty breathing, can sometimes be severe.
- Asthma may worsen.
Other Side Effects in Men
Very common (affects more than 1 in 10 people):
- Local reactions at the injection site, such as pain, redness, bruising, swelling, and/or irritation.
Common (affects up to 1 in 10 people):
- Swelling of the veins above and behind the testicles (varicocele).
- Development of breast tissue, acne, or weight gain.
Very rare (affects up to 1 in 10,000 people):
- Allergic reactions, such as skin rash, redness of the skin, blisters, swelling of the face with difficulty breathing, can sometimes be severe.
- Asthma may worsen.
Reporting of Side Effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing GONAL-f
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the cartridge or on the carton after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Before opening and within its validity period, the product can be stored outside the refrigerator at temperatures up to a maximum of 25°C for a single period of up to 3 months and must be discarded if not used within these 3 months.
Keep the cap on the pen to protect it from light.
Do not use GONAL-f if you notice any visible signs of deterioration, if the liquid contains particles, or if it is not transparent.
Once opened, the pen should be stored between 2°C and 25°C for a maximum of 28 days.
Do not use the medicine remaining in the pre-filled pen for more than 28 days.
At the end of the treatment, the unused solution should be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
GONAL-f Composition
- The active ingredient is folitropin alfa.
- Each pre-filled, multi-dose pen contains 900 IU (66 micrograms) of folitropin alfa in 1.44 ml of solution.
- The other components are poloxamer 188, sucrose, methionine, sodium dihydrogen phosphate monohydrate, disodium phosphate dihydrate, and m-cresol, as well as concentrated phosphoric acid and sodium hydroxide for pH adjustment and water for injectable preparations.
Product Appearance and Container Contents
- GONAL-f is presented as a clear, colorless injectable liquid in a pre-filled pen.
- It is supplied in boxes with 1 pre-filled pen and 16 disposable needles.
Marketing Authorization Holder
Merck Europe B.V., Gustav Mahlerplein 102, 1082 MA Amsterdam, Netherlands
Manufacturer
Merck Serono S.p.A., Via delle Magnolie 15, 70026 Modugno (Bari), Italy
Date of Last Revision of this Leaflet: {MM/YYYY}.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/.
Instructions for Use
GONAL-f PRE-FILLED PEN 900 IU/1.44 ml
Injectable solution in pre-filled pen
Folitropin alfa
Important Information about the GONAL-f Pre-filled Pen
- Read the instructions for use and the leaflet before using the GONAL-f pre-filled pen.
- Always follow all the instructions in these instructions for use and the training provided by the healthcare professional, as they may be different from those received previously. This information will help avoid errors in treatment or infections from needle sticks or glass breakage.
- The GONAL-f pre-filled pen is for subcutaneous injection only.
- Only use the GONAL-f pre-filled pen if the healthcare professional has shown you how to use it correctly.
- The healthcare professional will tell you how many GONAL-f pre-filled pens you need to complete your treatment.
- Take the injection at the same time every day.
- The numbers on the dose information windowrepresent the number of international units (IU) and show the dose of folitropin alfa. The healthcare professional will tell you how many IU of folitropin alfa to inject each day.
- The numbers shown on the dose information windowhelp you:
|
|
injection (Figure 2). |
|
injection with a second pen (Figure 3). |
|
- Remove the needle from the pen immediately after each injection.
Do notreuse needles.
Do notshare the pen or needles with anyone else.
Do notuse the GONAL-f pre-filled pen if it has been dropped or if the pen is cracked or damaged, as this may cause injury.
How to Use the GONAL-f Pre-filled Pen Treatment Diary
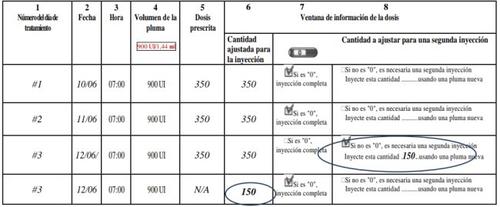
A treatment diary is included at the end of the instructions for use. Use the treatment diary to record the amount injected.
Injecting an incorrect amount of medication can affect treatment.
- Record the day of treatment (column 1), date (column 2), time of injection (column 3), and pen volume (column 4).
- Record the prescribed dose (column 5).
- Check that you have selected the correct dose before administering the injection (column 6).
- After the injection, read the number indicated on the dose information window.
- Confirm that you have received a complete injection (column 7) or record the number indicated on the dose information windowif it is different from "0" (column 8).
- When necessary, administer another injection with a second pen, selecting the remaining dose shown in the "Amount to adjust for a second injection" section (column 8).
- Record this remaining dose in the "Adjusted amount for injection" section (column 6) on the next line.
Using the treatment diary to record the daily injection(s) allows you to check that you have received the complete prescribed dose each day.
An example of a treatment diary:
Familiarize Yourself with the GONAL-f Pre-filled Pen
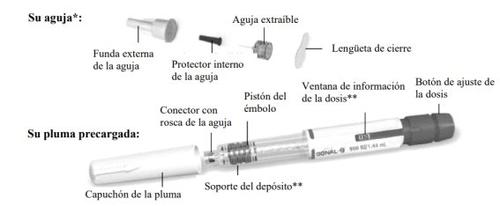
*For illustrative purposes only. The supplied needles may have a slightly different appearance.
**The numbers on the dose information windowand the cartridge holder represent the number of international units (IU) of the medication.
Step 1 Gather Materials
1.1 Leave the pre-filled pen at room temperature for at least 30 minutes before using it to allow the medication to reach room temperature. Do notuse a microwave or any other heating element to warm the pen. 1.2 Prepare a clean and flat surface, such as a table or countertop, in a well-lit area. 1.3 You will also need (not included in the packaging):
1.4 Wash your hands with soap and water and dry them thoroughly afterward (Figure 5). 1.5 Remove the GONAL-f pre-filled pen from the packaging with your hand. Do notuse any utensils, as this may damage the pen. 1.6 Check that the pre-filled pen says GONAL-f on it. 1.7 Check the expiration date on the pen label (Figure 6). Do notuse the GONAL-f pre-filled pen if it has passed the expiration date or if the pre-filled pen does not say GONAL-f. |
|
Step 2 Prepare for Injection
2.1 Remove the cap from the pen (Figure 7). 2.2 Check that the medication is clear and colorless and does not contain particles. Do notuse the pre-filled pen if the medication has changed color or is cloudy, as this may cause an infection. 2.3 Check that the dose information window is set to "0" (Figure 8). |
|
|
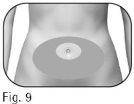
Choose an injection site: 2.4 The healthcare professional will indicate the injection sites to use around the stomach area (Figure 9). To minimize skin irritation, choose a different injection site each day. 2.5 Clean the skin at the injection site with an alcohol swab. Do nottouch or cover the skin that you have just cleaned. |
|
Step 3 Attach the Needle
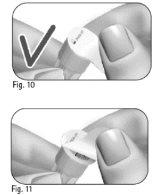
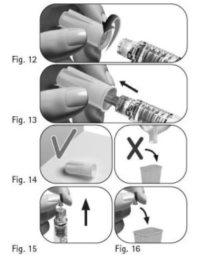
Important:always use a new needle for each injection. Reusing needles can cause infection. 3.1 Take a new needle. Use only the "single-use" needles supplied. 3.2 Check that the outer needle shield is not damaged. 3.3 Hold the outer needle shield firmly. 3.4 Check that the outer needle shield's closure tab is not damaged or loose and that it has not passed the expiration date (Figure 10). 3.5 Remove the closure tab (Figure 11). Do notuse the needle if it is damaged or expired or if the outer needle shield or closure tab is damaged or loose. Using expired or damaged needles can cause infection. Dispose of it in a sharps container and take a new needle. 3.6 Screw the outer needle shield onto the threaded tip of the GONAL-f pre-filled pen until you feel a slight resistance (Figure 12). Do notovertighten the needle when attaching it, as it may be difficult to remove after injection. 3.7 Remove the outer needle shield by gently pulling it (Figure 13). 3.8 Set it aside for later use (Figure 14). Do notdiscard the outer needle shield, as this will prevent needle stick injuries and infections when separating the needle from the pre-filled pen. 3.9 Hold the GONAL-f pre-filled pen with the needle pointing upward (Figure 15). 3.10 Carefully remove and discard the inner needle shield (Figure 16). Do notcover the needle with the inner needle shield again, as this may cause needle stick injuries and infections. 3.11 Examine the needle tip carefully for one or more drops of liquid (Figure 17).
|
|
If you do not see any drop of liquid on the needle tip or its surroundings the first time you use a new pen:
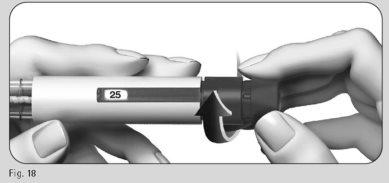
- Gently turn the dose adjustment button forward until it indicates "25" in the dose information window (Figure 18).
- You can turn the dose adjustment button backward if you have moved it beyond "25".
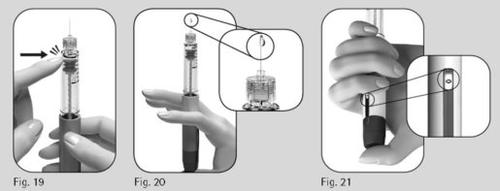
- Hold the pen with the needle pointing upward.
- Gently tap the cartridge holder (Figure 19).
- Press the dose adjustment button completely. A drop of liquid will appear on the needle tip (Figure 20).
- Check that the dose information window indicates "0" (Figure 21).
- Proceed with Step 4Choose the dose.
If a drop of liquid does not appear, contact the healthcare professional.
Step 4 Choose the Dose
Note:The pen contains 450 IU of folitropin alfa. The maximum single dose that can be adjusted in a 450 IU pen is 450 IU. The minimum single dose is 12.5 IU, and the dose can be increased in increments of 12.5 IU.
4.1Turn the dose adjustment button until the desired dose appears in the dose information window
- Example: if the desired dose is "150" IU, confirm that the dose information window shows "150" (Figure 22). Injecting an incorrect amount of medication can affect treatment.
|
|
|
|
4.2Check that the dose information windowindicates the complete prescribed dosebefore proceeding to the next step.
Step 5 Inject the Dose
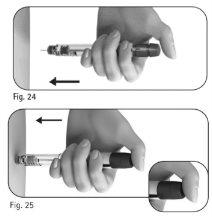
Important:inject the dose as instructed by the healthcare professional. 5.1 Slowly insert the entire needle into the skin (Figure 24). |
|
5.2 Place your thumb in the center of the dose adjustment button. Press the dose adjustment button slowly and completelyand hold it down to administer the injection (Figure 25). Note:the larger the dose, the longer it will take to inject. | |
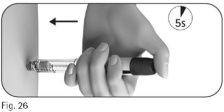
5.3 Hold the dose adjustment button down for at least 5 seconds before removing the needle from the skin (Figure 26).
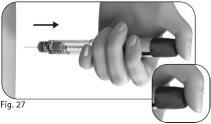
After a minimum of 5 seconds, remove the needle from the skin while holding the dose adjustment button down(Figure 27).
Do notrelease the dose adjustment button until you have removed the needle from the skin. |
|
Step 6 Remove the Needle after Each Injection
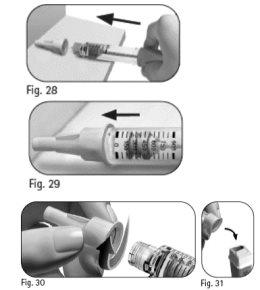
6.1 Place the outer needle shield on a flat surface. 6.2 Hold the GONAL-f pre-filled pen firmly with one hand and insert the needle into the outer needle shield (Figure 28). 6.3 Continue pushing the needle shield against a firm surface until you hear a "click" (Figure 29). 6.4 Hold the outer needle shield and unscrew the needle by turning it in the opposite direction (Figure 30). 6.5 Dispose of the used needle safely in a sharps container (Figure 31). Handle the needle with care to avoid injury. Do notreuse or share any used needle. |
|
Step 7 After Injection
7.1 Check that you have administered a complete injection:
If the dose information window shows "0", you have completed the dose. If the dose information window shows a number greater than "0", the GONAL-f pre-filled pen is empty. You have not received the complete prescribed dose and must perform the step 7.2 described below. |
|
7.2 Complete a partial injection (only when necessary):
To complete the dose with a second pen, repeat Steps 1 to 8. |
|
Step 8 Storing the GONAL-f Pre-filled Pen
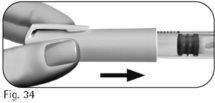
8.1 Replace the pen cap over the pen to avoid infection (Figure 34). 8.2 Store the pen with the cap on in a safe place and as indicated in the leaflet. 8.3 When the pen is empty, ask the healthcare professional |
How to dispose of it.
Do notkeep the pen with the needle still attached, as this can cause an infection.
Do notreuse the GONAL-f preloaded pen if it has fallen, or if the pen is cracked or damaged, as this can cause injury.
Contact your healthcare professional if you have any questions.
GONAL-f Preloaded Pen Treatment Diary
1 Treatment day number | 2 Date | 3 Time | 4 Pen volume | 5 Prescribed dose | 6 | 7 Dose information window | 8 |
Adjusted amount for injection | Amount to adjust for a second injection | ||||||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen | |||
/ | : | 900 IU | ?If it's a "0", complete injection | ?If it's not "0", a second injection is needed Inject this amount...using a new pen |
Date of the last revision of these instructions for use: {MM/YYYY}.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GONAL-F 900 IU/1.44 ML PRE-FILLED PEN INJECTION SOLUTIONDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INJECTABLE, 150 IU/0.25 ml (11 micrograms/0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for GONAL-F 900 IU/1.44 ML PRE-FILLED PEN INJECTION SOLUTION
Discuss questions about GONAL-F 900 IU/1.44 ML PRE-FILLED PEN INJECTION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions