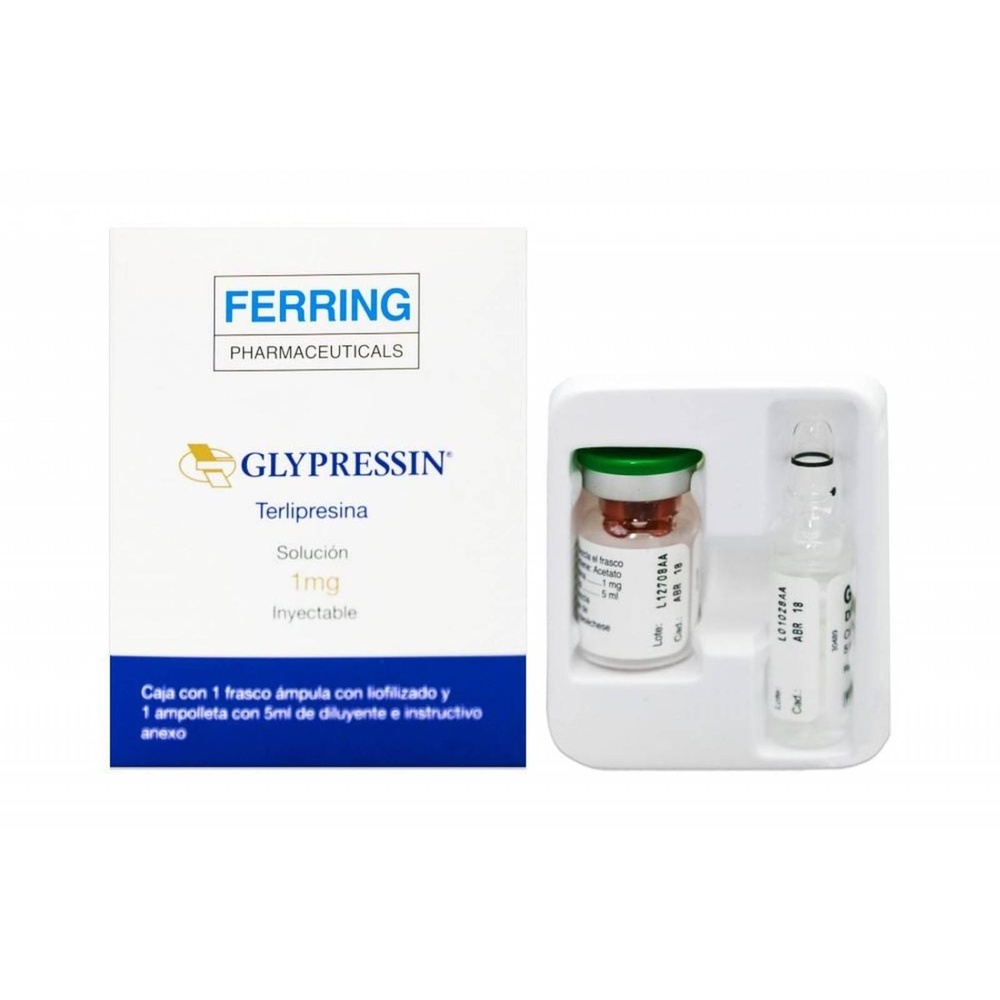
GLYPRESSIN 1 mg INJECTABLE SOLUTION

How to use GLYPRESSIN 1 mg INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Glypressin 1 mg solution for injection
Terlipressin acetate
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack:
- What Glypressin is and what it is used for
- What you need to know before you use Glypressin
- How to use Glypressin
- Possible side effects
- Storage of Glypressin
Contents of the pack and further information
1. What Glypressin is and what it is used for
Glypressin is a solution for intravenous injection, presented in a 8.5 ml glass ampoule containing 1 mg of terlipressin acetate (equivalent to 0.86 mg of terlipressin base). The concentration of the solution is 0.12 mg of terlipressin acetate/ml.
Glypressin (terlipressin) belongs to a group of medicines that decrease the pressure in the liver veins (portal venous pressure) in patients with high blood pressure in the vein that carries blood to the liver (portal hypertension). Terlipressin acts by constricting the blood vessels (vasoconstriction) in this area, helping to control bleeding from esophageal and stomach varices (esophagogastic) when it occurs.
Glypressin also contributes to improving blood circulation in the kidneys, helping to recover renal function in patients with Hepatorenal Syndrome (a type of kidney failure in patients with severe liver dysfunction)
Glypressin is indicated for the treatment of:
- Gastrointestinal bleeding due to rupture of esophagogastic varices
- Emergency treatment of hepatorenal syndrome type 1, defined according to the criteria of the CIA (International Ascites Club).
2. What you need to know before you start using Glypressin
Do not use Glypressin:
- if you are allergic (hypersensitive) to terlipressin acetate or any of the other components of this medicine (listed in section 6)
- if you are pregnant
Warnings and precautions:
Consult your doctor or pharmacist before starting treatment with Glypressin:
- if you have high blood pressure (hypertension).
- if you have heart problems, such as irregular heartbeat (arrhythmias), coronary artery disease, or your heart pumps less blood than it should (heart failure), as you are at greater risk of suffering from heart-related side effects.
- if you have cerebral vascular disease, peripheral vascular disease, or vascular disease of the intestines, as you are at greater risk of suffering from side effects related to lack of blood flow in these locations.
- if you have swelling of the legs due to poor circulation in the veins or are overweight (obesity), as you are at greater risk of suffering from decreased blood flow to the skin (ischemia) and even, in isolated cases, death of skin cells (necrosis).
- if you have a severe generalized infection with a drop in blood pressure (septic shock).
- if you have impaired renal function (renal insufficiency).
- if you have asthma or respiratory problems (respiratory insufficiency).
- in patients over 70 years of age with current or past cardiovascular disease.
- in children, as experience is limited in this age group.
Glypressin should be used under the supervision of a specialist in units with the availability to regularly monitor blood pressure, cardiac function, blood parameters, and fluid balance.
The injection should be administered exclusively by the intravenous routeto avoid local skin necrosis due to the product leaking into the skin.
Glypressin may increase the risk of developing respiratory failure, which can be fatal. If you experience difficulty breathing or symptoms of fluid overload, before or during treatment with Glypressin, inform your doctor immediately.
If you are being treated for a severe liver and kidney disease (hepatorenal syndrome type 1), your doctor should ensure that your cardiac function and fluid and electrolyte balance are monitored during treatment. Special care is required if you have pre-existing heart or lung disease, as Glypressin may induce cardiac ischemia (decreased blood flow to the heart) and respiratory failure (severe breathing difficulties). Treatment with Glypressin should be avoided if you have liver failure with multiple organ failures and/or renal insufficiency with very high levels of creatinine (a waste product) in the blood, as it increases the risk of adverse outcomes.
If you are being treated for a severe liver and kidney disease, Glypressin may increase the risk of developing sepsis (bacteria in the blood and extreme response of the body to an infection) and septic shock (a serious condition that occurs when a major infection causes low blood pressure and reduced blood flow). Your doctor will take additional precautions in your case.
Using Glypressin with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
It is very important to inform your doctor if you are being treated with:
- Betablockers (medicines to slow down the heart rate), as their effects may be increased if used at the same time as Glypressin.
- Antiarrhythmics (used to treat irregular heartbeats), such as quinidine or amiodarone.
- Diuretics (used to increase urine production, such as furosemide).
Tell your doctor if you have previously had a sudden slowing of the heart rate with certain anesthetics (propofol, sufentanil). Glypressin may increase the effect of these medicines if they are administered again.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Glypressin should not be administered if you are pregnant, as it may harm your baby.
Glypressin should not be administered during breastfeeding, as it is not known whether Glypressin can pass into breast milk.
Driving and using machines
No studies have been conducted on the effects of Glypressin on the ability to drive or use machines.
Use in children and over 65 years of age
Glypressin should be used with caution in patients over 70 years of age with current or past cardiovascular disease.
Special caution should be exercised when treating children, as experience is limited in this age group.
Use in patients with liver problems
In patients with liver problems, it is not necessary to adjust the dose of terlipressin.
Important information about some of the ingredients of Glypressin
Patients on low-sodium diets should note that this medicine contains 30.6 mg (1.33 mmol) of sodium per 1 mg of terlipressin acetate.
3. How to use Glypressin
Follow the instructions for administration of Glypressin exactly as indicated by your doctor. Consult your doctor or pharmacist if you have any doubts.
Method of use and route of administration
Administration of Glypressin should be carried out by qualified healthcare personnel.
Remove an ampoule from the packaging and ensure that no liquid remains in the head of the ampoule. Once the ampoule is opened, extract the solution with a syringe and inject intravenously.
The medicine, once opened, should be used immediately.
Glypressin is injected or infused intravenously.
The normal dose in adults is:
Gastrointestinal bleeding due to rupture of esophagogastic varices
It will be established by the doctor, depending on the patient's weight.
Generally, if the patient's weight is less than 50 kilograms, 1 milligram (1 ampoule of 8.5 ml) will be administered every four hours. In patients with a body weight between 50 and 70 kilograms, 1.5 milligrams (1.5 ampoules of 8.5 ml) will be administered every four hours. In patients with a body weight of more than 70 kilograms, 2 milligrams (2 ampoules of 8.5 ml) will be administered every 4 hours.
Treatment should be continued for 24 consecutive hours until bleeding has been controlled or for a maximum period of 48 hours. After the initial injection, subsequent doses may be reduced to 1 milligram (1 ampoule) of Glypressin when necessary, such as in the event of adverse reactions.
Hepatorenal syndrome
It is recommended to start treatment with 1 mg of terlipressin (1 ampoule) every 6 hours for at least 3 days. If after 3 days of treatment, the decrease in serum creatinine is less than 30% compared to the baseline value, it should be considered to double the dose to 2 mg (2 ampoules) every 6 hours.
Treatment with terlipressin should be discontinued if there is no response to treatment (defined as a decrease in serum creatinine of less than 30% on day 7 compared to the baseline value) or in patients with complete response (serum creatinine values below 1.5 mg/dl for at least two consecutive days).
In patients who present an incomplete response (decrease in serum creatinine of at least 30% compared to the baseline value but without reaching a value below 1.5 mg/dl on day 7), treatment with terlipressin may be maintained for up to a maximum of 14 days.
In the event of relapse of hepatorenal syndrome after a complete response, treatment with terlipressin may be restarted according to medical criteria.
The usual duration of treatment for hepatorenal syndrome is 7 days, and the maximum recommended duration is 14 days.
Hepatorenal syndrome type 1
Glypressin may also be administered as a drip (continuous intravenous infusion) starting normally with 2 mg of terlipressin acetate per day and increasing stepwise up to a maximum of 12 mg of terlipressin acetate per day.
If you use more Glypressin than you should
If you are administered more Glypressin than recommended, there is an increased risk of serious circulatory effects, including a hypertensive crisis.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, telephone 915 620 420, indicating the medicine and the amount ingested.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor or another healthcare professional immediately:
- If you develop breathing difficulties or experience a worsening of respiratory capacity (signs or symptoms of respiratory failure). This side effect is very common if you are being treated for hepatorenal syndrome type 1: it may affect more than 1 in 10 people.
- If you develop signs or symptoms of infection in the blood (sepsis/septic shock), which may include fever and chills or very low body temperature, pale or blue-tinged skin, severe shortness of breath, decreased urine production, rapid heartbeat, nausea and vomiting, diarrhea, fatigue and weakness, and dizziness. This side effect is common if you are being treated for hepatorenal syndrome type 1: it may affect up to 1 in 10 people.
Other side effects that may occur with different frequencies depending on the disease being treated.
Very common:may affect more than 1 in 10 people
If you have hepatorenal syndrome type 1:
- Difficulty breathing (dyspnea)
Common:may affect up to 1 in 10 people.
- Headache,
- Bradycardia (very slow heart rate).
- Increased blood pressure (hypertension).
- Peripheral vasoconstriction (inadequate blood flow to tissues) resulting in paleness.
- Transient stomach pain.
- Transient diarrhea.
If you have hepatorenal syndrome type 1:
- Fluid in the lungs (pulmonary edema)
- Breathing difficulties (respiratory difficulty)
Uncommon:may affect up to 1 in 100 people.
- Decreased sodium in the blood if fluid balance is not controlled
- Irrregular heartbeat
- Increased heart rate
- Chest pain
- Myocardial infarction (heart attack)
- Pulmonary edema
- Torsade de pointes (serious cardiac event)
- Heart failure. Symptoms include shortness of breath, fatigue, and swelling of the ankles
- Inadequate blood flow to the intestines
- Peripheral cyanosis (blue-tinged skin due to lack of oxygen)
- Hot flashes
- Breathing difficulties and respiratory failure (severe breathing difficulties)
- Transient nausea
- Transient vomiting
- Skin necrosis (tissue damage)
- Uterine contraction (contraction of the uterus)
- Decreased uterine blood flow
- Necrosis at the injection site (tissue damage)
If you have gastrointestinal bleeding due to rupture of esophagogastic varices
- Fluid in the lungs (pulmonary edema)
- Breathing difficulties (respiratory difficulty)
Rare:may affect up to 1 in 1,000 people.
If you have gastrointestinal bleeding due to rupture of esophagogastic varices:
- Difficulty breathing (dyspnea)
The antidiuretic effect of the medicine (decrease in urine production) may cause a decrease in sodium in the blood (hyponatremia) if fluid balance is not controlled.
Patient with Hepatorenal Syndrome treated with Glypressin presented during clinical trials a higher risk of suffering from cardiovascular side effects, such as decreased blood flow to the heart (myocardial ischemia), irregular heartbeat (arrhythmia), decreased blood flow to the intestines (intestinal ischemia), or circulatory overload (which may manifest as increased blood pressure, headache, difficulty breathing, or increased size of the neck veins).
During clinical trials and post-marketing experience, several cases of serious cardiac arrhythmias have been reported.
During post-marketing experience, several cases of skin ischemia and necrosis have been reported in areas of the skin distinct from the injection site of Glypressin.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: http://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Glypressin
Keep out of sight and reach of children.
Do not use Glypressin after the expiry date stated on the packaging, after "EXP". The expiry date is the last day of the month indicated. Store in a dry place, at a temperature between 2-8°C, protected from light. Do not freeze. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Glypressin 1 mg solution for injection:
The active substance is terlipressin acetate. Each ampoule, of 8.5 ml, contains 1 mg of terlipressin acetate (equivalent to 0.86 mg of terlipressin base). The concentration of the solution is 0.12 mg/ml terlipressin acetate.
The other ingredients are: sodium chloride, acetic acid, sodium acetate, and water for injection.
Appearance of the product and packaging contents
Glypressin 1 mg solution for injection is a clear and colorless solution presented in glass ampoules.
It is provided in packs of 5 ampoules, each containing 8.5 ml.
Marketing authorization holder and manufacturer
Marketing authorization holder:
FERRING S.A.U.
C/ del Arquitecto Sánchez Arcas nº3, 1º
28040 Madrid
SPAIN
Manufacturer:
FERRING-LECIVA, A.S.
K Rybniku 475 (Jesenice near Prague) –
252 42 - Czech Republic
FERRING GMBH
Wittland, 11-13 (Kiel) –
D-24109 – Germany
Date of last revision of this leaflet
January 2023
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GLYPRESSIN 1 mg INJECTABLE SOLUTIONDosage form: INJECTABLE, 1 mg terlipressin/ vialActive substance: terlipressinManufacturer: Ferring S.A.Prescription requiredDosage form: INJECTABLE, 0.2 mg/mlActive substance: terlipressinManufacturer: Ever Valinject GmbhPrescription requiredDosage form: INJECTABLE, 1 mgActive substance: terlipressinManufacturer: Altan Pharmaceuticals SaPrescription required
Online doctors for GLYPRESSIN 1 mg INJECTABLE SOLUTION
Discuss questions about GLYPRESSIN 1 mg INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions