
GAVISCON FORTE CHEWABLE TABLETS

How to use GAVISCON FORTE CHEWABLE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the user
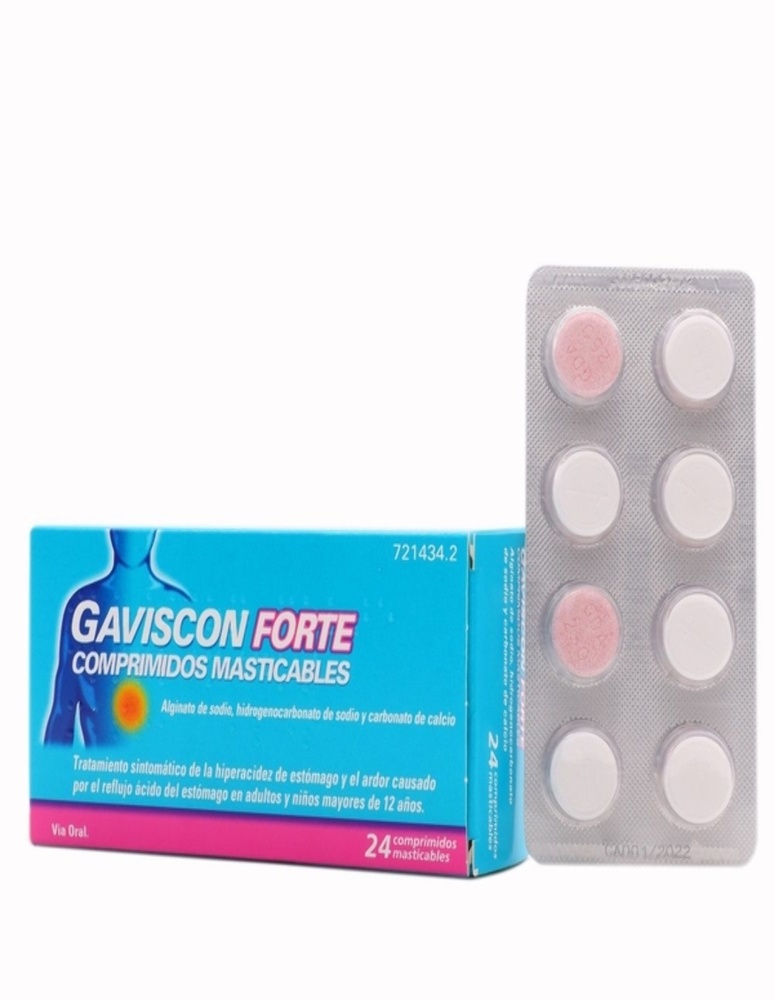
Gaviscon Forte chewable tablets
Sodium alginate, sodium hydrogen carbonate (sodium bicarbonate) and calcium carbonate
Read the entire leaflet carefully before starting to take this medicine, as it contains important information for you. Follow the administration instructions of the medicine contained in this leaflet or as indicated by your doctor or pharmacist.
|
Contents of the leaflet:
- What is Gaviscon Forte and what is it used for
- What you need to know before taking Gaviscon Forte
- How to take Gaviscon Forte
- Possible side effects
- Storage of Gaviscon Forte
- Contents of the pack and further information
1. What is Gaviscon Forte and what is it used for
It belongs to the group of medicines called other agents for peptic ulcer and gastroesophageal reflux.
It acts in two distinct ways:
- The sodium alginate together with sodium hydrogen carbonate (sodium bicarbonate) and calcium carbonate form a protective barrier in the stomach to prevent gastric reflux, calming the burning sensation in the stomach.
- The sodium hydrogen carbonate (sodium bicarbonate) and calcium carbonate neutralize excess acid in the stomach.
Gaviscon Forte is indicated for the symptomatic treatment of stomach hyperacidity and heartburn caused by acid reflux from the stomach in adults and adolescents over 12 years old.
You should consult a doctor if it worsens or does not improve after 7 days.
2. What you need to know before taking Gaviscon Forte
Do not take Gaviscon Forte:
- If you are allergic to calcium carbonate, sodium hydrogen carbonate (sodium bicarbonate), sodium alginate, or any of the other components of this medicine (listed in section 6).
- If you have severe kidney failure or suffer from kidney stones.
- If you have high levels of calcium in the blood or low levels of phosphates in the blood.
- If you have high levels of calcium in the urine.
Warnings and precautions
Consult your doctor or pharmacist before taking this medicine if you have mild or moderate kidney problems, have been diagnosed with sarcoidosis (inflammation that can affect multiple organs in the body), suffer from constipation or hemorrhoids, or suffer from symptoms of stomach or intestinal diseases, appendicitis, or edema.
If you are taking or are going to take other medicines, you should separate their intake by 1 to 2 hours (see "Other medicines and Gaviscon Forte").
Avoid prolonged use as it can cause kidney stones, as well as high doses over a long period, which can also cause high levels of calcium in the blood or urine, kidney failure, or worsen it if you already have it.
The medicine should not be taken with milk or dairy products.
If the symptoms persist after 7 days of treatment, consult your doctor.
Children and adolescents
Gaviscon Forte is not recommended for children under 12 years old.
Interference with laboratory tests
If you are going to have any laboratory tests (including blood tests, urine tests, skin tests that use allergens, etc.), inform your doctor that you are taking this medicine, as it may alter the results.
Other medicines and Gaviscon Forte
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medicine.
Before taking this medicine, inform your doctor if you are taking antibiotics (tetracyclines, quinolones), heart medicines such as cardiac glycosides (digoxin, digitoxin), or other medicines such as fluorides, phosphates, iron salts, ketoconazole, neuroleptics, thyroid hormones, penicillamine, glucocorticoids, chloroquine, bisphosphonates, beta-blockers (atenolol, metoprolol, propranolol), or estramustine, as it may affect the efficacy of these medicines.
As Gaviscon Forte may interfere with some medicines, after taking it, you should wait 2 hours before taking another medicine orally. If you have taken another medicine, before taking Gaviscon Forte, you should wait 1 to 2 hours to get the maximum benefit of the treatment with that other medicine.
Taking Gaviscon Forte with food and drinks
Like all antacids that contain calcium, this medicine should not be taken with large amounts of milk or dairy products, as it may cause an increase in blood calcium levels and milk-alkali syndrome (Burnett syndrome).
Fertility, pregnancy, and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
This medicine can be used during pregnancy and breastfeeding if taken according to these recommendations and not taken for a long time.
As this medicine provides a significant amount of calcium in addition to what the pregnant or breastfeeding woman takes every day, pregnant or breastfeeding women should not exceed the amounts indicated in the section 3. How to take Gaviscon Forteand should not take large amounts of dairy products and milk at the same time (1 liter of milk contains 1.2 grams of elemental calcium).
Driving and using machines
This medicine does not affect the ability to drive or use machines.
Gaviscon Forte contains sodium, aspartame, azorubine, and mannitol.
This medicine contains 223.56 mg of sodium (main component of table salt/cooking salt) in each 4 tablets. This is equivalent to 11.2% of the maximum recommended daily sodium intake for an adult.
Consult your doctor or pharmacist if you need 7 or more tablets per day for a prolonged period, especially if you have been advised to follow a low-salt diet (sodium).
This medicine contains 23.44 mg of aspartame in each 4 tablets.
Aspartame contains a source of phenylalanine, which may be harmful in cases of phenylketonuria (PKU), a rare genetic disease in which phenylalanine accumulates because the body is unable to eliminate it properly.
This medicine may cause allergic reactions because it contains azorubine (E-122). It can cause asthma, especially in patients allergic to acetylsalicylic acid.
It may have a mild laxative effect because it contains mannitol.
3. How to take Gaviscon Forte
Follow the administration instructions of the medicine contained in this leaflet or as indicated by your doctor or pharmacist. In case of doubts, ask your doctor or pharmacist.
The recommended dose in adults, including elderly people and adolescents over 12 years old, is 2 to 4 tablets, as needed, 1 hour after meals and before bedtime. You can take a maximum of 16 tablets per day.
Kidney failure: caution is required in patients with low-salt diets (see section 2).
How to take
This medicine is taken orally.
Remove the tablets from the packaging and chew them.
If the symptoms persist after 7 days of continuous treatment, consult your doctor to rule out more serious diseases.
If you take more Gaviscon Forte than you should
Overdose symptoms include nausea and vomiting, constipation, fatigue, increased urine production, thirst, dehydration, and abnormal muscle weakness.
Drink plenty of water and consult your doctor or pharmacist.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or go to a medical center or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to take Gaviscon Forte
Do not take a double dose to make up for the forgotten doses.
When you need it, take it again as indicated in the section 3. How to take Gaviscon. If you have any other doubts about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
During the use of the association of sodium alginate, sodium hydrogen carbonate (sodium bicarbonate), and calcium carbonate, the following side effects have been observed with the following frequencies:
Frequency not known (frequency cannot be estimated from available data):
- Allergic reactions, such as skin rashes and itching, difficulty breathing, and swelling of the face, mouth, or throat, and anaphylactic shock.
- Increased calcium levels in the blood, especially in people with kidney function disorders (with prolonged use and high doses).
- Constipation, nausea, vomiting, fatigue, confusion, increased urine production, thirst, and dehydration (alkalosis) (with prolonged use and high doses).
- Milk-alkali syndrome (Burnett syndrome) that can cause high levels of calcium in the blood (with prolonged use and high doses).
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency (www.notificaram.es). By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Gaviscon Forte
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging after CAD. The expiration date is the last day of the month indicated.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and medicines you no longer need in the pharmacy's SIGRE point. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Gaviscon Forte
The active ingredients are 250 mg of sodium alginate, 106.5 mg of sodium hydrogen carbonate (sodium bicarbonate), and 187.5 mg of calcium carbonate.
The other ingredients (excipients) are macrogol (20000), mannitol (E-421), copovidone, acesulfame potassium (E-950), aspartame (E-951), peppermint flavor, azorubine (E-122), magnesium stearate, xylitol DC (contains sodium carmellose).
Appearance of the product and contents of the pack
Gaviscon Forte are chewable tablets, flat, and circular. One layer of the tablet is pink and slightly speckled, and the other is white.
They are presented in PVC/PE/PVdC strips, transparent, and unprinted with an aluminum foil in a cardboard box with its leaflet.
Each pack can contain 4, 24, 32, 48, and 64 tablets.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Reckitt Benckiser Healthcare, S.A.
C/ Mataró, 28
08403 Granollers-Barcelona
Spain
RB NL Brands B.V. Schiphol Blvd 207, 1118 BH Schiphol, Netherlands
Date of the last revision of this leaflet: August 2020.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GAVISCON FORTE CHEWABLE TABLETSDosage form: CAPSULE, 300 mgManufacturer: Laboratorios Vinas S.A.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 300 mgManufacturer: Laboratorios Vinas S.A.Prescription requiredDosage form: TABLET, Bismuth Subcitrate 120 mgActive substance: bismuth subcitrateManufacturer: Tora Laboratories S.L.U.Prescription required
Online doctors for GAVISCON FORTE CHEWABLE TABLETS
Discuss questions about GAVISCON FORTE CHEWABLE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















