FOSTIPUR KIT 300 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION


How to use FOSTIPUR KIT 300 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fostipur Kit300 UIpowder and solvent for injectable solution
Urofollitropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Fostipur Kit is and what it is used for
- What you need to know before you use Fostipur Kit
- How to use Fostipur Kit
- Possible side effects
- Storing Fostipur Kit
- Contents of the pack and other information
1. What Fostipur Kit is and what it is used for
- Fostipur Kit is used to stimulate ovulation in women who do not ovulate and who do not respond to other treatments (clomiphene citrate).
in the induction of multifollicular development (and, consequently, multiple eggs) in women undergoing fertility treatments.
- In
Women undergoing fertility treatments.
Urofollitropin is a highly purified follicle-stimulating hormone that belongs to a group of medicines called gonadotropins.
This medicine should be used under the supervision of your doctor.
2. What you need to know before you use Fostipur Kit
Before starting treatment, the fertility of the couple should be assessed.
Do not use Fostipur Kit
- If you are allergic to urofollitropin or any of the other ingredients of this medicine (listed in section 6).
- Enlargement of the ovaries or cysts in the ovary not due to a hormonal disorder (polycystic ovary syndrome).
Bleeding of unknown origin.
- Ovarian, uterine, or breast cancer.
- Abnormal swelling (tumor) of the pituitary gland or hypothalamus (in the brain).
You should not use this medicine if you have conditions such as premature menopause, malformation of the sexual organs, or tumors of the uterus that prevent a normal pregnancy.
Warnings and precautions
Although there is no information on allergic reactions with Fostipur Kit, you should inform your doctor if you have any allergic reactions to similar medicines.
This treatment increases the risk of developing a disease known as ovarian hyperstimulation syndrome (OHSS) (see Possible side effects). If ovarian hyperstimulation occurs, you should stop treatment and avoid becoming pregnant. The first signs of ovarian hyperstimulation are pain in the lower abdomen, as well as nausea, vomiting, and weight gain. If these symptoms appear, you should be examined by your doctor as soon as possible. In severe cases, although rare, the ovaries may increase in size and fluid may accumulate in the abdomen or chest.
The medicine used to achieve the final release of mature eggs (which contains human chorionic gonadotropin, hCG) may increase the likelihood of OHSS. Therefore, it is not advisable to use hCG in cases where ovarian hyperstimulation is developing, and you should not have sexual intercourse, even using barrier contraceptive methods, for at least 4 days.
It should be noted that women with fertility problems have a higher rate of spontaneous abortions than the normal population.
The occurrence of multiple pregnancies and births in patients receiving ovulation induction treatment increases compared to natural conception. However, this risk can be reduced if the recommended dose is used.
There is a slight increase in the risk of ectopic pregnancy in women with damaged Fallopian tubes.
Multiple pregnancies and characteristics of parents undergoing fertility treatments (e.g., mother's age, sperm characteristics) may be associated with a higher risk of birth defects.
Treatment with Fostipur Kit, like pregnancy itself, may increase the risk of thrombosis. Thrombosis is the formation of a blood clot in a blood vessel, most often in the veins of the legs or in the lungs.
Consult your doctor before starting treatment, especially:
This medicine is prepared from human urine. The risk of transmission of infection or disease to the body cannot be completely eliminated. However, this risk is limited by the virus elimination phases in the manufacturing process, particularly AIDS, Herpes virus, and Papillomavirus.
No cases of viral contamination have been reported.
Using Fostipur Kit with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
Fostipur Kit should not be used if you are pregnant or breastfeeding.
Fostipur Kit contains sodium
This medicine contains less than 1mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
3. How to use Fostipur Kit
Dose and duration of treatment:
Follow your doctor's administration instructions for this medication exactly. If in doubt, consult your doctor or pharmacist again.
Women who do not ovulate and have irregular or incomplete menstrual periods:
If you have your menstrual period, treatment should be started within 7 days following the onset of menstruation (the first 7 days of the menstrual cycle).
The dose consists of 1 injection per day, under the skin (subcutaneously).
The common initial dose is 75 IU to 150 IU of FSH (Fostipur Kit) per day. This dose may be increased if necessary, by 37.5 to 75 IU at 7-day or, preferably, 14-day intervals, to obtain an adequate response.
The maximum daily dose of FSH should not generally exceed 225 IU.
If your doctor does not find an adequate response after 4 weeks of treatment, this treatment cycle should be discontinued. For the next cycle, your doctor will indicate a treatment with a higher initial dose.
When a good response is obtained (satisfactory follicular growth), you will be given only one injection of another medication (hCG), used to induce follicular maturation and ovulation release. This will take place 24-48 hours after the last injection of Fostipur Kit. It is recommended to have sexual intercourse on the same day of hCG administration and the next day.
If an excessive ovarian response is obtained, treatment should be discontinued and hCG should not be administered (see Possible adverse effects). For the next cycle, your doctor will indicate a lower initial dose.
Women undergoing ovarian stimulation for multiple follicular development prior to in vitro fertilization or other assisted reproduction techniques:
Situation 1 - If you have your menstrual period
Treatment should be started 2 or 3 days after the onset of your menstrual period (the first 2 or 3 days of the menstrual cycle).
The dose consists of 1 injection per day, subcutaneously.
A commonly used dose for superovulation consists of administering 150 to 225 IU of Fostipur Kit per day. Treatment continues, with dose adjustment according to your response, until adequate follicular development is achieved. This is usually achieved on the 10th day of treatment (an average of 5 to 20 days) and is evaluated by blood sampling and/or ultrasound examinations.
The maximum dose is generally 450 IU/day.
Once follicular development is achieved, a single injection of a medication used to induce final follicular maturation will be administered; this medication contains up to 10,000 IU of human chorionic gonadotropin (hCG). It will be administered 24-48 hours after the last injection of Fostipur Kit.
Oocyte puncture will be performed approximately 35 hours later.
Situation 2 - When a gonadotropin-releasing hormone (GnRH) agonist is used
Fostipur Kit should be administered approximately 2 weeks after the start of this treatment. Both treatments are maintained until adequate follicular development is achieved. An injection of Fostipur Kit will be administered per day, subcutaneously. For example, after 2 weeks of treatment with a GnRH agonist, 150 to 225 IU of Fostipur Kit will be administered for the first 7 days. The dose will then be adjusted according to the ovarian response.
Administration instructions:
Fostipur Kit is administered by injection under the skin (subcutaneously).
Each vial is for single use and the injection should be administered immediately after preparation.
After advising and practicing conveniently, your doctor may ask you to administer the Fostipur Kit injection yourself.
First, your doctor should:
- Let you practice administering the subcutaneous injection yourself.
- Indicate to you which areas are possible for you to administer the injection.
- Indicate to you how to carefully prepare the solution for injection.
- Explain to you how to prepare the correct dose to be administered.
Before you administer the Fostipur Kit injection, carefully read the following instructions:
How to prepare and inject 1 vial of Fostipur Kit, using 1 vial of powder:
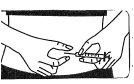
The solution should be prepared just before administering the injection. Each vial is for single use. The medication should be reconstituted under aseptic conditions.
Fostipur Kit should be reconstituted only with the solvent provided in the kit.
Prepare a clean surface and wash your hands before reconstituting the solution. It is essential that both your hands and the utensils you will use are as clean as possible.
Lay out the following materials on a clean surface:
- 2 pieces of cotton with alcohol (not included in the box),
- 1 vial containing Fostipur Kit powder,
- 1 pre-filled syringe with solvent,
- 1 needle for preparing the injection,
- 1 fine needle for subcutaneous injection.
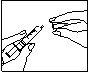
Reconstitution of the injection solution using 1 vial of powder.
Preparing the injection solution:
|
|
|
|
|
DO NOT SHAKE, but gently movethe vial between your hands until the powder is completely dissolved, trying to avoid creating foam. |
|
Preparation of higher doses, using more than 1 vial of powder
If your doctor has recommended higher doses, you can achieve this by using more than 1 vial of powder with a pre-filled syringe of solvent.
When reconstituting more than 1 vial of Fostipur Kit, at the end of phase 4 described above, introduce the reconstituted contents of the first vial back into the syringe, and slowly inject it into a second vial. Repeat phases 2 to 4 for the second vial and subsequent ones until the contents of the required number of vials equivalent to the prescribed dose are dissolved (within the limit of the maximum total dose of 450 IU, corresponding to a maximum of 6 vials of Fostipur Kit 75 IU, 3 vials of Fostipur Kit 150 IU, or 2 vials of Fostipur Kit 225 IU).
Your doctor may increase your dose by 37.5 IU, which represents half a vial of Fostipur Kit 75 IU.
To do this, you must reconstitute the contents of the 75 IU vial according to phases 2 to 3 described above and introduce half of this reconstituted solution (0.5 ml) into the syringe according to phase 4.
In that situation, you will have two preparations to inject: the first reconstituted preparation in 1 ml and the second containing 37.5 IU in 0.5 ml.
Both preparations should be injected with their own syringes according to the following phases.
The solution must be transparent and colorless.
Inject the medication subcutaneously:
|
|
|
|
The injection site:
- Your doctor or nurse will have indicated to you which part of your body you can inject the medication into.
The most common placesarethe thigh orthe lower abdominal wallbelow the navel.
- Clean the injection site with an alcohol swab.
Placing the needle:
|
|
Injecting the solution:
- Inject it under the skin as you were taught. Do not inject it directly into a vein. Push the plunger slowly and constantly, so that the solution is injected correctly and the skin is not damaged.
Take all the timeyou needto inject the prescribed volume of the solution. As described in the preparation of the solution, depending on the dose prescribed by your doctor, you may not need to use the total volume of the solution.
Removing the needle:
- Quickly remove the syringe and press on the injection site with an antiseptic swab. A gentle massage at the site, while still maintaining pressure, helps to disperse the Fostipur Kit solution and alleviate discomfort.
Disposal of all used utensils:
Any unused product or waste material should be disposed of according to local requirements (once the injection is finished, all needles and empty syringes should be discarded in an appropriate container).
If you use more Fostipur Kit than you should
The effects of an overdose of Fostipur Kit are unknown, although it is likely that an ovarian hyperstimulation syndrome (see Possible adverse effects) could occur. If you administer more Fostipur Kit than you should, contact your doctor or pharmacist.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or the Toxicology Information Service, phone: 915 620 420, indicating the medication and the amount ingested.
If you forget to use Fostipur Kit
Take the next injection at the scheduled time. Do not use a double dose to make up for forgotten doses.
If you interrupt treatment with Fostipur Kit
Do not interrupt treatment on your own initiative. Always consult your doctor if you are considering stopping the use of this medication. If you have any other doubts about the use of this medication, consult your doctor or pharmacist.
4. Possible adverse effects
Like all medications, Fostipur Kit can cause adverse effects, although not everyone will experience them.
The following adverse effects are important and will require immediate action if you experience them.
You should discontinue the administration of Fostipur Kit and visit your doctor immediately if the following occurs:
Frequent, may affect 1 to 10 out of 100 people:
- Ovarian Hyperstimulation Syndrome (see Section 2 of the additional information)
The following adverse effects have also been reported:
Frequent, may affect 1 to 10 out of 100 people:
- Headache,
- feeling of swelling in the abdomen,
- constipation,
- pain at the injection site.
Uncommon, may affect 1 to 10 out of 1000 people:
- Increased activity of the thyroid gland,
- mood changes,
- fatigue,
- dizziness,
- difficulty breathing (dyspnea),
- nosebleeds,
- nausea, indigestion, abdominal pain,
- redness, itching,
- flushing,
- cystitis,
- breast enlargement, breast pain,
- difficulty stopping bleeding.
Redness, pain, and hematoma at the injection site may occur (frequency not established).
See section 2 of the additional information on the risk of blood clots, ectopic pregnancy, multiple pregnancies, and miscarriages.
If you consider that any of the adverse effects you are experiencing is serious or if you notice any adverse effect not mentioned in this leaflet, inform your doctor or pharmacist.
Reporting of adverse effects:
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Fostipur Kit
Keep this medication out of sight and reach of children.
Do not store above 25°C. Keep the vial and the pre-filled syringe of solvent in the outer packaging to protect it from light.
Do not use this medication after the expiration date shown on the carton and on the vial.
Use immediately after reconstitution.
Do not use Fostipur Kit if you notice that the solution does not appear transparent. After reconstitution, the solution should be transparent and colorless.
Medications should not be thrown away through wastewater or household waste. Deposit the packaging and medications you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Fostipur Kit
The active ingredient is urofolitropin.
Each vial contains 300 IU of urofolitropin (follicle-stimulating hormone FSH): 1 ml of reconstituted solution contains 300 IU of urofolitropin.
The specific activity in vivois equal to or greater than 5000 IU of FSH per mg of protein.
Other components are:
Powder: lactose monohydrate.
Solvent: sodium chloride and water for injectable preparations.
Appearance of the product and package contents
Fostipur Kit is presented as a powder and solvent for injectable solution.
Boxes with 1, 5, or 10 kits. Each kit contains: 1 vial with powder containing 300 IU of urofolitropin, 1 pre-filled syringe with 1 ml of solvent, 1 needle for reconstitution, and 1 needle for subcutaneous injection.
The appearance of the powder is a hardened white or off-white mass, and the solvent is transparent and colorless.
Marketing authorization holder and manufacturer
Marketing authorization holder
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia 2
26900 Lodi (Italy)
Manufacturer
IBSA Farmaceutici Italia Srl., Via Martiri di Cefalonia, 2 - 26900 Lodi (Italy)
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (Spain)
This medication is authorized in the Member States of the European Economic Area with the following names (concentrations and pharmaceutical forms are identical in all countries, only trade names differ)
Austria: Fostimon PFS
Belgium: Fostimon
Cyprus: Fostimon PFS
Denmark: Fostimon Set
Finland: Fostimon Set
France: Fostimonkit
Luxembourg: Fostimon
Ireland: Fostimon PFS
Netherlands: Fostimon Set
Norway: Fostimon Set
Spain: Fostipur Kit
Sweden: Fostimon Set
United Kingdom: Fostimon PFS
Date of the last revision of this leaflet:February 2024
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FOSTIPUR KIT 300 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 150 IU urofolitropin/ mlActive substance: urofollitropinManufacturer: Ibsa Farmaceutici Italia S.R.L.Prescription requiredDosage form: INJECTABLE, 75 IU per 1 ml of solution / 5000 IU of FSH per mgActive substance: urofollitropinManufacturer: Ibsa Farmaceutici Italia S.R.L.Prescription requiredDosage form: INJECTABLE, 150 IU per 1 mL of reconstituted solutionActive substance: urofollitropinManufacturer: Ibsa Farmaceutici Italia S.R.L.Prescription required
Online doctors for FOSTIPUR KIT 300 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about FOSTIPUR KIT 300 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





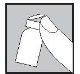

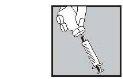 With the needle still inserted, turn the vial upside down.
With the needle still inserted, turn the vial upside down.