
FOSCAN 1 mg/ml INJECTABLE SOLUTION

How to use FOSCAN 1 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Foscan 1 mg/ml Solution for Injection
Temoporfin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Foscan and what is it used for.
- What you need to know before you use Foscan.
- How to use Foscan.
- Possible side effects
- Storage of Foscan
- Contents of the pack and other information
1. What is Foscan and what is it used for
The active substance of Foscan is temoporfin.
Foscan is a porphyrin photosensitising medicine for the treatment called photodynamic therapy, which increases sensitivity to light and is activated by light using a laser.
Foscan is used for the treatment of head and neck cancer in patients who cannot be treated with other therapies.
2. What you need to know before you use Foscan
Do not use Foscan:
- if you are allergic to temoporfin or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic (hypersensitive) to porphyrins,
- if you have porphyria, or any other disease that worsens with light,
- if the tumour being treated is close to a major blood vessel,
- if you are going to have surgery in the next 30 days,
- if you have an eye condition that requires an examination with bright light in the next 30 days,
- if you are already being treated with a photosensitising agent.
Warnings and precautions
- Foscan will make you sensitive to light for about 15 days after injection. This means that normal daylight or intense artificial light can cause burns to your skin. To prevent this, you mustcarefully follow the instructions for gradual exposure to increasing levels of indoor light during the first week and filtered and dim outdoor light during the second week after treatment (see the table at the end of this leaflet).
- Please discuss this with your doctor after receiving an injection of Foscan.
- Sunscreen creams do notprevent this sensitivity.
- Gradually, you will lose sensitivity to light. Normally, people can start to receive normal outdoor light after 15 days.
- For 30 days after treatment with Foscan, do notallow an optician or ophthalmologist to examine your eyes with bright lights.
- For 3 months after treatment with Foscan, do notuse UV tanning beds or sunbathe.
- For 6 months after treatment with Foscan, avoid prolonged exposure to direct sunlight on the arm where you received the injection. As a precaution, if you plan to engage in prolonged outdoor activities, protect the injection site using long-sleeved and coloured clothing.
The following table of instructions indicates what you should do to avoid skin burns. You must follow these instructions carefully.
If you have any doubts, ask your doctor or nurse.
Time after Foscan injection | What should you do to avoid burns? |
Day 1 (0-24 hours) | Stay indoors in a dark room. Keep curtains closed and use 60W or less light bulbs. Avoid direct sun exposure. |
Days 2-7 | You can gradually return to using normal indoor light. Remember to avoid direct sunlight coming through windowsor direct light from household appliances such as reading lamps. You can watch TV. You can go outside after sunset If you absolutely need to go outside during daylight hours, you must cover all your skin, including your face and hands, and wear dark glasses. The type of clothing you should wear is:
During this week, you may have very sensitive eyes to bright lights. You may feel eye or headache pain when lights are turned on. If you have problems, wear sunglasses. |
Days 8-14 | You can now start going outside during daylight hours. Stay in areas with dim or cloudy light. Continue to wear dark, dense clothing. Start on day 8 with 10-15 minutes outside. If you do not observe any skin redness in the following 24 hours, you can gradually increase the time you spend outside during the week. Avoid direct sunlight or intense artificial light. Stay in the shade. |
From day 15 onwards | Your sensitivity to light will gradually return to normal. You mustcarefully test this by exposing the back of your hand to sunlight for 5 minutes. Wait 24 hours to see if there is any redness. If there is redness, you must avoid direct sunlight for another 24 hours. You can then repeat the test. If there is no redness, you can gradually increase your exposure to sunlight day by day. Do not stay in the sun for more than 15 minutes the first time. Most people will be able to return to their normal routine by day 22. The first day after the skin test, you can stay in direct sunlight for 15 minutes. You can increase your exposure by another 15 minutes each day, i.e. 30 minutes on the second day, 45 minutes on the third day, 60 minutes on the fourth day, and so on. If at any time you feel a stinging or burning sensation, or observe skin redness after sun exposure, wait until it disappears before exposing your skin to light again during this period. For 30 days after treatment with Foscan, avoid vision tests that use bright lights. For 3 months after treatment with Foscan, avoid UV tanning beds. Do notsunbathe. For 6 months after treatment with Foscan, avoid prolonged exposure to direct sunlight on the arm where you received the injection. As a precaution, if you plan to engage in prolonged outdoor activities, protect the injection site using long-sleeved and coloured clothing. |
Using Foscan with other medicines
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
- Avoidbecoming pregnant during the 3 months following treatment with Foscan.
- Consult your doctor or pharmacist before using any medicine if you are pregnant.
Do notbreastfeed until at least 1 month after the injection of Foscan.
Driving and using machines
- The amount of alcohol in this medicine may affect your ability to drive or use machines.
- Do notdrive or use machines during the first 15 days after the injection of Foscan, due to the recommended restrictions on light exposure.
Foscan contains ethanol (alcohol)
- This product contains 48 vol. % ethanol by volume (alcohol), i.e. up to 4.2 g per dose, equivalent to 84 ml of beer, 35 ml of wine per dose. This product is harmful to individuals with alcoholism. It should be taken into account in the case of pregnant or breastfeeding women, children, and at-risk groups such as patients with liver disease or epilepsy.
The amount of alcohol in this medicine may alter the effects of other medicines.
3. How to use Foscan
- Your doctor or nurse will administer Foscan by slow injection into a vein, which will take approximately 6 minutes.
- Four days after the injection, your doctor will treat your cancer with laser light. Your doctor will cover the normal tissue surrounding your cancer and then direct the laser light at the cancer for about 5 minutes. The laser light is not hot and does not burn.
If you are given more Foscan than you should
- It may not be possible to apply the laser treatment.
- You may be sensitive to light for more than 15 days.
You must carefully follow the instructions to avoid skin burns.
If you have any further questions on the use of this product, ask your doctor or nurse.
4. Possible side effects
Like all medicines, Foscan can cause side effects.
- Everyone who uses Foscan becomes sensitive to light for about 15 days after the injection.
- You must follow the instructions given to you to avoid sunlight and intense artificial light.
- These instructions are written in this leaflet. Your doctor will also advise you on what to do.
If you do not follow these instructions, you may suffer from severe sunburn that can cause permanent scarring.
Most side effects related to photodynamic therapy are local effects observed as a result of the activation of Foscan with the laser. You may feel pain after laser treatment. This pain will be controlled with painkillers. Tell your doctor or nurse if you have pain or if the painkillers they have given you do not relieve the pain. Additionally, you may observe swelling and redness around the treated area. You may be given medication to reduce the swelling. After 2 to 4 days, the treated area will become black. This is due to the dead cancer cells (necrosis). Foscan can also damage the tissue surrounding the tumour.
Very common side effects(may affect more than 1 in 10 people):
- You may feel pain when Foscan is injected.
- After laser treatment, you may feel pain in the treated area, e.g. pain in the face or headache.
- Additionally, bleeding, ulcers, swelling in the treated area such as facial or tongue swelling, and scarring may occur.
- You may experience constipation.
You may have difficulty eating or drinking due to these effects.
Common side effects(may affect up to 1 in 10 people):
- Irritation, burning sensation, or skin damage may occur at the injection site, but it will not last long.
- Ulcers, blisters, redness, or darkening of the skin may also occur.
- Vomiting
- Fever
- Nausea
- Anaemia
- Photosensitivity
- Sunburn
- Burns
- Difficulty swallowing
- Dizziness
- You may notice swelling or stiffness in the jaw. Some people may develop an infection in the treated area, e.g. mouth or throat inflammation.
Frequency not known (cannot be estimated from the available data):
- Blockage of the airways due to swelling of the treated area
- Fistula in the treated area
- Sepsis
- Vascular rupture
Severe side effects such as inflammation of the bile ducts or gallbladder, liver abscess, or perforation in the treated area have been reported with the use of Foscan in tumours other than head and neck. Ask your doctor for more information.
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Foscan
Keep this medicine out of the sight and reach of children.
Do notuse this medicine after the expiry date which is stated on the label and carton. The expiry date is the last day of the month stated.
Foscan will be stored in the hospital pharmacy.
Do notstore above 25°C.
Store in the original packaging to protect from light. Once the medicine is removed from the container, it must be used immediately.
Each vial is a single dose and any unused medicine should be discarded.
6. Container Contents and Additional Information
Composition of Foscan
- The active ingredient is temoporfin. Each ml contains 1 mg of temoporfin.
- The other components are anhydrous ethanol (E1510) and propylene glycol (E1520).
Appearance of the Product and Container Contents
Foscan injectable solution is a dark purple solution presented in an amber glass vial, containing 1 ml, 3 ml, or 6 ml of solution.
Each container contains a glass vial and a filter.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
biolitec Pharma Ltd.
Otto-Schott-Str. 15
07745 Jena
Germany
Phone: +49 3641 5195330
Fax: +49 3641 5195331
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu
This information is intended solely for medical professionals or healthcare professionals:
Foscan 1 mg/ml Solution for Injection
Temoporfin
- CONTAINER CONTENTS
The active substance is temoporfin. Each ml of solution contains 1 mg of temoporfin. The excipients are anhydrous ethanol and propylene glycol. A filter with Luer connections for syringe and cannula is provided.
Each container contains a vial with 1 ml, 3 ml, or 6 ml of injectable solution.
Each vial is a single dose, and the unused solution must be discarded.
- DOSE CALCULATION
Calculate the required dose of Foscan according to the patient's body weight. The dose is 0.15 mg/kg of body weight.
- ADMINISTRATION OF FOSCAN (96 hours before laser illumination of the treatment site)
Foscan must be administered intravenously through a resident cannula in a large proximal vein of an extremity, preferably in the antecubital fossa. The patency of the resident cannula must be tested before administering the injection.
The dark purple color of the solution, along with the amber vial, makes it impossible to perform a visual check for particles. For this reason, an in-line filter must be used as a precautionary measure, which is included in the packaging.
Collect all the contents of the vial containing Foscan with a syringe and expel the air (Figure 1).

Attach the filter to the syringe (Figure 2).
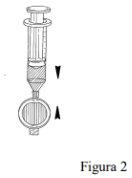
Press the syringe plunger to fill the entire dead space within the filter. Continue pressing the plunger to expel excess Foscan until the required volume remains in the syringe, including enough to cover the dead space of the intravenous cannula (Figure 3).
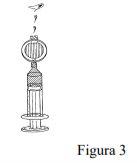
Attach the syringe and filter to the cannula. Administer the required dose of Foscan by slow intravenous injection over at least 6 minutes (Figure 4).

Remove the intravenous cannula immediately after injection. DO NOT flush with aqueous solutions such as sodium chloride injection solution 9 mg/ml (0.9%) or water for injectable preparations.
Special care must be taken to avoid extravasation at the injection site. If extravasation occurs, the exposed area must be protected from light for a period of at least 3 months. There is no known benefit to injecting another substance into the extravasation site.
Foscan is photosensitive. Once removed from its vial, it must be administered immediately. When a delay is unavoidable, the solution must be protected from light.
- LASER ILLUMINATION OF THE TREATMENT SITE
Please consult the user manual for the laser and microlens fiber optic.
96 hours after administration of Foscan, the treatment site must be illuminated with 652 nm light from an approved laser source. The light must be applied to the entire surface of the tumor using an approved microlens fiber optic. Whenever possible, the illuminated area should extend beyond the tumor margin by a distance of 0.5 cm.
Light must not be administered before 90 hours or after 110 hours of Foscan injection.
The incident light dose is 20 J/cm2, applied by the microlens fiber optic in a circular field to the surface of the tumor with an irradiance of 100 mW/cm2, which implies an illumination time of 200 seconds.
Each field must be illuminated only once per treatment. Multiple non-overlapping fields can be illuminated. It must be ensured that no region of tissue receives a light dose greater than the specified dose. Tissues outside the target area must be completely protected to avoid photoactivation by scattered or reflected light.
- SAFETY INFORMATION
Foscan is not irritant.
Disposal of unused medicinal products and all materials that have come into contact with them must be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FOSCAN 1 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 1 mgActive substance: temoporfinManufacturer: Biolitec Pharma LimitedPrescription requiredDosage form: INJECTABLE, 1 mg/mlActive substance: temoporfinManufacturer: Biolitec Pharma LimitedPrescription requiredDosage form: GEL, 78 mg aminolevulinic acid/gActive substance: aminolevulinic acidManufacturer: Biofrontera Bioscience GmbhPrescription required
Online doctors for FOSCAN 1 mg/ml INJECTABLE SOLUTION
Discuss questions about FOSCAN 1 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






