
FORMODUAL 200 micrograms / 6 micrograms / actuation pressurized solution for inhalation

How to use FORMODUAL 200 micrograms / 6 micrograms / actuation pressurized solution for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Formodual 200micrograms/6micrograms/puff inhalation solution in a pressurised container.
dipropionate of beclometasone/formoterol fumarate dihydrate
For use in adults
Read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Formodual and what is it used for
- What you need to know before you use Formodual
- How to use Formodual
- Possible side effects
- Storing Formodual
- Contents of the pack and other information
1. What is Formodual and what is it used for
Formodual is an inhalation solution in a pressurised container that contains two active substances that are inhaled through the mouth and released directly into the lungs.
The two active substances are:
Beclometasone dipropionate, which belongs to a group of medicines called corticosteroids that have an anti-inflammatory action that reduces inflammation and irritation in your lungs.
Formoterol fumarate dihydrate, which belongs to a group of medicines called long-acting bronchodilators that relax the muscles in the airways, making it easier for you to breathe.
These two active substances combined make breathing easier. They also help prevent asthma symptoms such as difficulty breathing, wheezing, and coughing.
Formodual is used to treatasthma in adults.
If you have been prescribed Formodual, it is likely that:
- Your asthma is not adequately controlled with inhaled corticosteroids and short-acting bronchodilators used as needed, or
- Your asthma responds well to a combination treatment of corticosteroids and long-acting bronchodilators.
2. What you need to know before you use Formodual
Do not use Formodual:
- If you are allergic to beclometasone dipropionate or formoterol fumarate dihydrate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Tell your doctor, pharmacist or nurse before you start using Formodual if you have:
- heart problems, such as angina (chest pain), heart failure, narrowing of the arteries, heart valve disease, or any other known heart abnormality
- high blood pressure or if you know you have an aneurysm (an abnormal bulge in the blood vessel wall)
- heart rhythm disorders, such as increased heart rate or irregular heartbeat, rapid pulse or palpitations, or if you have been informed that you have electrocardiographic abnormalities
- overactive thyroid gland
- low potassium levels in the blood
- any liver or kidney disease
- diabetes (inhalation of high doses of formoterol may cause an increase in blood glucose levels. When you start using this medicine and from time to time during treatment, you will need to have additional blood tests to check your blood sugar levels)
- adrenal gland tumour (called phaeochromocytoma)
Always consult your doctor before you start using Formodual if you are in any of these situations.
If you have or have had medical problems or allergies, or if you are not sure if you can use Formodual, consult your doctor, asthma nurse specialist or pharmacist before you start using this medicine.
Your doctor may want to check your blood potassium levels from time to time, especially if you have severe asthma.Like many other bronchodilators, Formodual may cause a sudden drop in blood potassium levels (hypokalaemia). This is because a lack of oxygen in the blood combined with other treatments you may be taking with Formodual can worsen the decrease in potassium levels.
If you are taking high doses of inhaled corticosteroids for long periods, you may need more corticosteroids in stressful situations. These situations include hospitalisation after an accident, severe injury, or before surgery. In this case, the doctor treating you will decide whether it is necessary to increase the dose of corticosteroids and may prescribe steroid tablets or injections.
In case you need to go to hospital,remember to take all your medicines and inhalers, including Formodual and any other medicines or tablets you have bought without a prescription, with you to the hospital, if possible in their original packaging.
Contact your doctor if you experience blurred vision or other visual disturbances.
Children and adolescents
Formodual should not be used in children and adolescents under 18 years of age.
Other medicines and Formodual
Tell your doctor, pharmacist or nurse if you are using or have recently used any other medicines, including those obtained without a prescription. This is because Formodual may affect how some medicines work. Also, some medicines may affect how Formodual works.
In particular, tell your doctor or pharmacist if you are using any of the following medicines:
- Some medicines may increase the effects of Formodual, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
- Beta-blocker medicines. Beta-blockers are medicines used to treat many conditions, such as heart problems, high blood pressure, or glaucoma (increased eye pressure). If you need to use beta-blockers (including eye drops), the effect of formoterol may be reduced or even cancelled.
- Beta-adrenergic medicines (medicines with the same action as formoterol) may increase the effects of formoterol.
- Medicines for treating heart rhythm disorders (quinidine, disopyramide, procainamide).
- Medicines for treating allergic reactions (antihistamines such as terfenadine).
- Medicines for treating depression or psychiatric disorders such as monoamine oxidase inhibitors (e.g. phenelzine, isocarboxazid) or tricyclic antidepressants (e.g. amitriptyline and imipramine), or phenothiazines.
- Medicines for treating Parkinson's disease (levodopa).
- Medicines for treating hypothyroidism (levothyroxine).
- Medicines containing oxytocin (which causes uterine contractions).
- Medicines used to treat psychiatric disorders such as monoamine oxidase inhibitors (MAOIs), including drugs with similar properties, such as furazolidone and procarbazine.
- Medicines for treating heart conditions (digoxin).
- Other medicines used to treat asthma (theophylline, aminophylline or steroids).
- Diuretics.
Tell your doctor if you are going to have a general anaesthetic for a surgical or dental procedure.
Pregnancy, breastfeeding and fertility
There are no clinical data on the use of Formodual during pregnancy.
Do not use Formodual if you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, unless your doctor tells you to.
Driving and using machines
Formodual is unlikely to affect your ability to drive or use machines.
Formodual contains alcohol
Formodual contains 9 mg of alcohol (ethanol) in each puff, which is equivalent to 0.25 mg/kg per dose of two puffs. The amount in two puffs of this medicine is equivalent to less than 1 ml of wine or beer. The small amount of alcohol in this medicine has no noticeable effect.
3. How to use Formodual
Use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Your doctor will regularly check that you are using the optimal dose of Formodual. Your doctor will adjust the treatment to the minimum dose that best controls your symptoms.
Dose:
Adults and elderly patients:The recommended dose is two puffs twice a day.
The maximum daily dose is 4 puffs.
Remember: you should always carry your quick-acting "rescue" inhaler with you in case your asthma symptoms get worse or in case you have a sudden asthma attack.
Patients at risk
No dose adjustment is needed in elderly patients. There is no information on the use of Formodual in patients with liver or kidney problems.
Use in children and adolescents (under 18 years of age)
Children and adolescents under 18 years of age should NOT use this medicine.
Formodual is effective for the treatment of asthma at a dose of beclometasone dipropionate that may be lower than that of other inhalers containing the same component. If you have previously been using another inhaler containing beclometasone dipropionate, your doctor will advise you on the exact dose of Formodual you should take for your asthma.
Do not increase the dose
If you think the medicine is not working very well, always talk to your doctor before increasing the dose.
If your asthma gets worse
If your symptoms get worse or become difficult to control (for example, if you use your quick-acting "rescue" inhaler more often), or if your quick-acting "rescue" inhaler does not improve your symptoms, see your doctor immediately. Your asthma may be getting worse, and your doctor may need to change the dose of Formodual or prescribe an alternative treatment.
Method of administration:
Formodual is for inhalation use only.
This medicine is in a pressurised container inside a plastic casing with a mouthpiece.
There is a dose counter on the back of the inhaler, which shows how many doses are left. Each time you press the canister, a puff of medicine is released, and the counter counts down one dose. Avoid dropping the inhaler, as this may cause the counter to count down a dose.
Checking the inhaler
Before using the inhaler for the first time, or if you have not used it for 14 days or more, you must check your inhaler to make sure it is working correctly.
- Remove the mouthpiece cover.
- Hold the inhaler upright with the mouthpiece at the bottom.
- Point the mouthpiece away from you and press the canister firmly to release a dose.
- Check the dose counter. If you are checking your inhaler for the first time, the counter should show 120.

How to use your inhaler
When possible, stand or sit upright to take the inhalation.
Before you start the inhalation, check the dose counter: any number between "1" and "120" shows that there are doses left. If the dose counter shows "0", there are no doses left - dispose of the inhaler and get a new one.
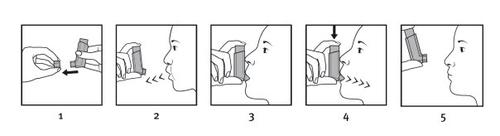
- Remove the mouthpiece cover and check that it is clean, i.e. that there are no dust, dirt or other foreign particles.
- Breathe out as slowly and deeply as possible.
- Hold the canister upright with the body facing upwards and place the mouthpiece between your lips. Do not bite the mouthpiece.
- Inhale slowly and deeply through your mouth and, just as you start to inhale, press the top of the inhaler firmlyto release a dose. If you have weakness in your hands, you may find it easier to hold the inhaler with both hands: place your two index fingers on the top of the inhaler and your two thumbs on the bottom of the inhaler.
- Hold your breath for as long as possible and finally remove the inhaler from your mouth and breathe out slowly. Do not blow into the inhaler.
If you need to take another dose, hold the inhaler upright for about half a minute and repeat steps 2 to 5.
Important:do not perform steps 2 to 5 too quickly.
After administration, replace the mouthpiece cover and check the dose counter.
To reduce the risk of fungal infections of the mouth and throat, rinse your mouth or gargle with water or brush your teeth after using the inhaler.
You should have a replacement inhaler when the counter shows the number 20. Stop using the inhaler when the counter shows 0, as although there may be puffs left in the device, they may not be enough to take a full dose.
If some of the gas escapes from the top of the inhaler or from the corner of your lips, it means that Formodual will not reach your lungs as it should. Take another dose following the instructions, starting again from step 2.
If you think the effect of Formodual is too strong or too weak, talk to your doctor or pharmacist.
If you find it difficult to press the inhaler while starting to breathe in, you can use the AeroChamber Plus spacer device. Ask your doctor, pharmacist or nurse about this device.
It is important that you read the leaflet that comes with the AeroChamber Plus spacer device and that you follow the instructions for use and cleaning carefully.
Cleaning
You should clean the inhaler once a week.
When you clean it, do not remove the pressurised canister from the device and do not use water or other liquids to clean the inhaler.
To clean the inhaler:
- Remove the mouthpiece cover from the inhaler.
- Wipe the inside and outside of the mouthpiece and device with a clean, dry cloth or paper tissue.
- Replace the mouthpiece cover.
If you use more Formodual than you should
- If you use more formoterol than you should, you may experience the following effects: nausea, vomiting, rapid heartbeat, palpitations, heart rhythm disorders, certain electrocardiographic abnormalities (heart tracing), headache, tremors, drowsiness, excess acid in the blood, low potassium levels in the blood, and high glucose levels in the blood. Your doctor may ask you to have blood tests to check your potassium and glucose levels in your blood.
- Taking too much beclometasone dipropionate may cause short-term changes in the functioning of the adrenal glands. This situation will improve in a few days; however, your doctor may need to check your cortisol levels in your blood.
Talk to your doctor if you experience any of these symptoms.
If you forget to use Formodual
Take it as soon as you remember. However, if it is almost time for your next dose, skip the missed dose and only take the next dose at the scheduled time. Do not take a double dose to make up for forgotten doses.
If you stop using Formodual
Even if you feel better, do not stop taking Formodual or reduce the dose. If you want to do so, talk to your doctor. It is very important that you use Formodual regularly, even if you do not have symptoms.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine may cause adverse effects, although not all people suffer from them.
As with other inhaler treatments, there is a risk of worsening breathing difficulties and wheezing immediately after using Formodual, which is known as paradoxical bronchospasm. If this happens, SUSPEND the use of Formodual immediatelyand use your rapid-acting "rescue" inhaler to treat breathing difficulties and wheezing symptoms. Contact your doctor immediately.
Contact your doctor immediatelyif you experience hypersensitivity reactions, such as skin allergies, itching, rash, skin redness, swelling of the skin or mucous membranes, especially in the eyes, face, lips, and throat.
Other possible adverse effects are listed below according to their frequency.
Frequent(may affect 1 in 10 patients):
- fungal infections (of the mouth and throat)
- headache
- hoarseness
- sore throat
Infrequent(may affect 1 in 100 patients):
- palpitations, exceptionally rapid heartbeat, and heart rhythm disorders
- certain electrocardiographic alterations (ECG)
- increased blood pressure
- flu-like symptoms
- sinusitis
- rhinitis
- ear inflammation
- throat irritation
- cough and productive cough
- asthma attack
- vaginal fungal infections
- nausea
- alteration or decrease in taste
- lip burning
- dry mouth
- difficulty swallowing
- indigestion
- stomach discomfort
- diarrhea
- muscle pain and cramps
- redness of the face and throat
- increased blood flow in certain tissues of the body
- excessive sweating
- tremors
- restlessness
- dizziness
- rash or hives
- alterations in certain blood components:
- decrease in white blood cell count
- increase in platelet count
- decrease in blood potassium concentration
- increase in blood sugar
- increase in insulin, free fatty acids, and ketone bodies in blood
The following adverse effects have also been reported as "infrequent" in patients with chronic obstructive pulmonary disease:
- pneumonia; inform your doctor if you experience any of the following symptoms: increased sputum production, changes in sputum color, fever, increased cough, increased respiratory problems
- reduction of cortisol in the blood; this is caused by the effect of corticosteroids on the adrenal gland
- irregular heartbeats
Rare(may affect 1 in 1,000 patients):
- chest tightness
- feeling of missed heartbeats (due to premature contraction of the heart ventricles)
- decrease in blood pressure
- kidney inflammation
- persistent swelling of the skin and mucous membranes for several days
Very Rare(may affect 1 in 10,000 patients):
- difficulty breathing
- worsening of asthma
- decrease in platelet count
- swelling of hands and feet
Inhalation of corticosteroids in high doses for a prolonged period may rarely cause systemic effects,including:
- problems with the functioning of the adrenal glands (suppression of adrenal function)
- decrease in bone mineral density (weakening of bones)
- growth retardation in children and adolescents
- increased intraocular pressure (glaucoma)
- cataracts
Frequency not known (cannot be estimated from available data):
- sleep problems
- depression or anxiety
- nervousness
- overexcitement or irritability
These effects may occur more frequently in children, but their frequency is unknown.
- Blurred vision
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is an adverse effect that does not appear in this prospectus.
You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Formodual
Keep this medicine out of sight and reach of children.
For the pharmacist:
Store in a refrigerator at 2°C to 8°C for a maximum period of 18 months.
For the patient:
Do not use this medicine after 3 months from the date you acquired the inhaler at the pharmacy and never use it after the expiration date that appears on the box and label. The expiration date is the last day of the indicated month.
Do not store the inhaler at a temperature above 25°C.
If the inhaler has been exposed to intense cold, warm it with your hands for a few minutes before use. Never heat it by artificial means.
Warning: the container contains a pressurized liquid. Do not expose the container to temperatures above 50°C or puncture it.
Medicines should not be thrown away through drains or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Formodual
The active ingredients are: beclometasone dipropionate and formoterol fumarate dihydrate.
Each dose of the inhaler contains 200 micrograms of beclometasone dipropionate and 6 micrograms of formoterol fumarate dihydrate. This corresponds to a released dose from the mouthpiece of 177.7 micrograms of beclometasone dipropionate and 5.1 micrograms of formoterol fumarate dihydrate.
The other components are: norflurane (HFC-134a), anhydrous ethanol, hydrochloric acid.
This medicine contains fluorinated greenhouse gases.
Each inhaler contains 10.356 g of norflurane (HFC-134a), which corresponds to 0.015 tons of CO2 equivalent (global warming potential GWP = 1430).
Appearance of the Product and Package Contents
Formodual is an inhalation solution in a pressurized container contained in an aluminum-coated container with a dosing valve, equipped with a plastic actuator that incorporates a dose counter (120-dose container) or a dose indicator (180-dose container), with a protective cap also made of plastic.
Each package contains:
1 pressurized container (providing 120 puffs)
2 pressurized containers (each providing 120 puffs)
1 pressurized container (providing 180 puffs)
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
CHIESI ESPAÑA, S.A.U.
Plaza de Europa, 41-43, 10th floor
08908 L’Hospitalet de Llobregat
Barcelona (Spain)
Batch Release Manufacturer:
Chiesi Farmaceutici S.p.A.
Via San Leonardo 96
43122 Parma - Italy
or
Chiesi Pharmaceuticals GmbH
Gonzagagasse 16/16
1010 Vienna - Austria
or
Chiesi S.A.S.
2 rue des Docteurs Alberto et Paolo Chiesi
41260 La Chaussée Saint-Victor
France
This medicine is authorized in the following Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following trademarks:
France, Spain, United Kingdom: Formodual
Germany: Kantos Master 200 micrograms/6 micrograms per inhalation pressurized inhalation solution
Belgium, Luxembourg, Greece: Inuvair
Bulgaria, Lithuania, Latvia, Estonia, Cyprus, Romania: Foster
Italy: Inuver
Denmark, Finland: Innovair
Norway: Inuxair
Date of the last revision of this prospectus:November 2023.
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Average pharmacy price44.8 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FORMODUAL 200 micrograms / 6 micrograms / actuation pressurized solution for inhalationDosage form: PULMONARY INHALATION, 100 micrograms/6 micrograms/actuationActive substance: formoterol and beclometasoneManufacturer: Cipla Europe N.V.Prescription requiredDosage form: PULMONARY INHALATION, 200 micrograms/6 micrograms/actuationActive substance: formoterol and beclometasoneManufacturer: Cipla Europe N.V.Prescription requiredDosage form: PULMONARY INHALATION, 100 micrograms/6 micrograms/actuationActive substance: formoterol and beclometasoneManufacturer: Lupin Europe GmbhPrescription required
Online doctors for FORMODUAL 200 micrograms / 6 micrograms / actuation pressurized solution for inhalation
Discuss questions about FORMODUAL 200 micrograms / 6 micrograms / actuation pressurized solution for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















