
FLUTIFORM 250 micrograms / 10 micrograms INHALATION SUSPENSION in pressurized container

How to use FLUTIFORM 250 micrograms / 10 micrograms INHALATION SUSPENSION in pressurized container
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Flutiform and what is it used for
- What you need to know before you use Flutiform
- Do not use Flutiform if:
- How to use Flutiform
- Follow the instructions for administration of this medicine exactly as directed by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
- Possible Adverse Effects
- Storage of Flutiform
- Package Contents and Additional Information
- Composition of Flutiform
Introduction
Package Leaflet: Information for the User
Flutiform 250 micrograms/10 micrograms/inhalation, inhalation suspension in pressurised container
Fluticasone propionate/formoterol fumarate dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.,as it contains important information for you..
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Flutiform and what is it used for
- What you need to know before you use Flutiform
- How to use Flutiform
- Possible side effects
- Storing Flutiform
- Contents of the pack and further information
1. What is Flutiform and what is it used for
Note:
The name of the medicine is Flutiform inhalation suspension in pressurised container, however, throughout this leaflet it is shortened to Flutiform. Sometimes a specific dose is referred to.
Flutiform is an inhaler (an inhalation suspension in pressurised container) that contains two active substances:
- Fluticasone propionate, which belongs to a group of medicines called steroids. Steroids help to reduce swelling and inflammation in the lungs.
- Formoterol fumarate dihydrate, which belongs to a group of medicines called long-acting beta2 agonists. Long-acting beta2 agonists are long-acting bronchodilators that help to keep the airways in the lungs open, making it easier to breathe.
These two active substances together help to improve breathing. You should use this medicine every day, as directed by your doctor.
This medicine helps to prevent breathing problems such as asthma and helps to prevent shortness of breath and breathing difficulties.However, it does not work if you are already having an asthma attack, i.e. if you are already short of breath and wheezing. If this happens, you will need to use a "rescue" medicine that works quickly, such as salbutamol.
2. What you need to know before you use Flutiform
Do not use Flutiform if:
- you are allergic to fluticasone propionate, formoterol fumarate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you start using Flutiform.
Before starting treatment with this inhaler, tell your doctor or pharmacist or nurse if you:
- Have tuberculosis (TB) now or have had it in the past. Symptoms include a persistent cough, often with bloody mucus, fever, tiredness, loss of appetite, weight loss and night sweats.
- Have a lung or chest infection.
- Have heart problems, such as problems with blood flow to the heart or narrowing of one of the heart valves (the aortic valve), heart failure, which can cause difficulty breathing or swelling of the ankles, a condition in which the heart muscle is enlarged (hypertrophic cardiomyopathy), an irregular heartbeat (cardiac arrhythmias) or if you have been told that your heart's electrocardiogram is abnormal (prolonged QTc interval).
- Have an abnormal bulge in the wall of a blood vessel (aneurysm).
- Have diabetes.
- Have high blood pressure.
- Have an overactive thyroid gland, which can cause increased appetite, weight loss or sweating (thyrotoxicosis).
- Have low blood potassium levels, which can cause muscle weakness, muscle spasms or an abnormal heart rhythm (hypokalaemia).
- Have adrenal gland problems (if your adrenal gland is not working properly, you may have symptoms such as headaches, weakness, tiredness, abdominal pain, loss of appetite, weight loss, dizziness, low blood pressure, diarrhoea, feeling unwell or fits) or an adrenal gland tumour (phaeochromocytoma).
- Have liver problems.
Tell your doctor if you experience blurred vision or other visual disturbances.
If you are going to have surgery or are under a lot of stress, tell your doctor, as you may need extra steroid treatment to control your asthma.
Other medicines and Flutiform
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including those obtained without a prescription. If you use this inhaler with other medicines, the effect of this inhaler or the other medicine may be altered.
Tell your doctor or pharmacist if you are taking the following medicines:
- Medicines known as beta-blockers (such as atenolol for high blood pressure, sotalol for irregular heart rhythms, metoprolol for fast heart rate or timolol in eye drops for glaucoma).
- Other medicines used to treat asthma or breathing problems (such as theophylline or aminophylline).
- Medicines that contain adrenaline or similar substances (including other beta agonists such as salbutamol or beta blockers such as atenolol, metoprolol, propranolol, timolol). Other long-acting beta2 agonists must not be used with this inhaler. If your asthma gets worse between doses of Flutiform, you should use your quick-acting "rescue" inhaler to get immediate relief.
- Medicines for treating allergic reactions (antihistamines).
- Medicines for treating high blood pressure or fluid build-up by increasing the amount of urine produced (diuretics).
- Medicines used to treat heart failure (such as digoxin).
- Medicines for treating irregular heart rhythms (such as quinidine, disopyramide, procainamide).
- Medicines for treating symptoms of depression or mental disorders, such as monoamine oxidase inhibitors (e.g. phenelzine and isocarboxazid), tricyclic antidepressants (e.g. amitriptyline and imipramine), or if you have taken any of these types of medicines in the last two weeks.
- Medicines used to treat psychiatric or mental disorders (phenothiazines or antipsychotics).
- Other medicines that contain steroids.
- Antifungal medicines (such as ketoconazole or itraconazole).
- Some medicines may increase the effects of Flutiform and your doctor may want to monitor you closely if you are taking these medicines (including some medicines for HIV: ritonavir, atazanavir, indinavir, nelfinavir, saquinavir or cobicistat).
- Antibiotics (such as clarithromycin, telithromycin or furazolidone).
- Medicines for treating Parkinson's disease (levodopa).
- Medicines for treating underactive thyroid gland (levothyroxine).
- Medicines for treating Hodgkin's disease (procarbazine).
- Medicines for inducing labour (oxytocin).
If you are going to have an operation under general anaesthesia, please tell your doctor at the hospital that you are using this inhaler.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Your doctor will advise you whether you should use this medicine.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use machines.
Flutiform contains ethanol (alcohol) and sodium cromoglicate
This medicine contains 2 mg of alcohol in each dose (2 inhalations). The amount in each dose is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine does not have any noticeable effects. It also contains a very small amount of sodium cromoglicate, however, patients who are currently taking cromoglicate (used to treat asthma, allergic rhinitis and allergic conjunctivitis) should continue to take it as normal.
3. How to use Flutiform
Follow the instructions for administration of this medicine exactly as directed by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
You should use your inhaler regularly, i.e. two inhalations (puffs) in the morning and two inhalations (puffs) in the evening, every day, in order to get the most benefit from your inhaler, unless your doctor advises you otherwise or recommends that you stop treatment. Do not take more doses than prescribed. Your doctor may have prescribed your inhaler for a different indication than asthma or a different dose than that prescribed normally and as described in this leaflet. You should always use your inhaler exactly as your doctor has recommended. If you are not sure how much to take or how often to use the inhaler, consult your doctor or pharmacist.
Adults, adolescents and children 5 years and older
The usual dose is two inhalations twice a day, i.e. two inhalations (puffs) in the morning and two in the evening. Your doctor will prescribe the dose needed to treat your asthma. Only adultsshould use the higher dose inhaler (Flutiform 250 micrograms/10 micrograms).
Only adults and adolescents over 12 years of age should use the medium dose inhaler (Flutiform 125 micrograms/5 micrograms).
Flutiform should not be used in children under 5 years of age.
Instructions for use
Read the leaflet carefully before using the inhaler and follow the instructions for use in the text and diagrams shown below. Your doctor or pharmacist will show you how to use your inhaler correctly. The medicine is inside, in an aerosol canister (see Diagram 1) which is inside a plastic dispenser (also known as an actuator). The actuator also has a counter to show how many inhalations (puffs) are left, after it has been loaded. This counter also has a colour code. It starts with green, then when there are less than 50 puffs (inhalations) left it changes to yellow and when there are less than 30 puffs (inhalations) left it changes to red. When it is near zero, you should contact your doctor to replace the inhaler. Do not use your inhaler when the counter shows zero.
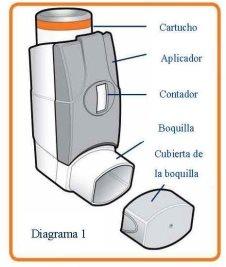
Before using your inhaler for the first time, or if you have not used it for more than 3 days, or if it has been exposed to freezing conditions
If your inhaler is new or you have not used it for more than 3 days, you must load it to ensure that it works correctly and that you get the correct dose.
If your inhaler has been exposed to freezing temperatures, you must let it come to room temperature for 30 minutes and then load it to ensure that it works correctly and that you get the correct dose.
To load the inhaler
- Remove the mouthpiece cover and shake the inhaler well.
- Point the mouthpiece away from you and release a puff (by pressing down the aerosol canister). This step must be repeated 4 times.
The inhaler must always be shaken immediately before use.
Using the inhaler
If while using Flutiform you feel that you are having difficulty breathing or wheezing, you should continue to use Flutiform, but go to see your doctor as soon as possible, as you may need additional treatment. Once your asthma is well controlled, your doctor may consider it possible to gradually reduce the dose of Flutiform.
Slowly carry out steps 2 to 5 shown below.
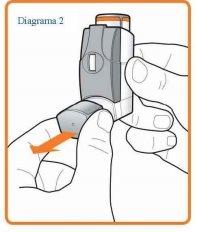
- Remove the mouthpiece cover (see Diagram 2) and check that your inhaler is clean and free from dust.
- Shake the inhaler immediately before each puff to ensure that the contents of your inhaler are evenly mixed.
- Sit up straight or stand up. Breathe out as slowly and deeply as you can.
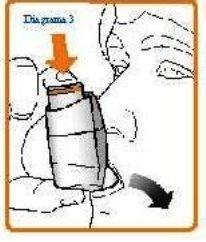
- Hold your inhaler upright (as shown in Diagram 3) and put the mouthpiece in your mouth, surrounding it with your lips. Hold the inhaler with your thumb(s) on the base of the mouthpiece and your index finger(s) on the top of the inhaler. Do not bite the mouthpiece.
- Breathe in slowly and deeply through your mouth and, at the same time, press down the aerosol canister to release a puff. Continue to breathe in steadily and deeply (if possible for about 2-3 seconds for children and 4-5 seconds for adults).

- Hold your breath, then take the inhaler out of your mouth. Continue to hold your breath for as long as you comfortably can. Do not breathe out into the inhaler.
- For the second puff, keep the inhaler upright, then repeat steps 2 to 6.
- Replace the mouthpiece cover.
You can practice in front of a mirror. If when you use your inhaler you see a "fine mist" coming out of the top of the inhaler or around your mouth, you may not have inhaled the medicine correctly. Take another dose, repeating the process from step 2 above.
After each inhalation, always rinse your mouth, gargle with water or brush your teeth and spit out the residue. This may help to prevent the risk of developing sores in the mouth and throat or hoarseness.
If you have weak hands, you may find it easier to hold the inhaler with both hands, putting your two index fingers on the aerosol canister and both thumbs on the base of the inhaler.
If you have difficulty using your inhaler, your doctor may provide you with a device called an AeroChamber Plus Flow-Vu spacer device, to help you get the medicine into your lungs properly. Your doctor or pharmacist will inform you about how to use the AeroChamber Plus Flow-Vu spacer device with your inhaler. The AeroChamber Plus Flow-Vu spacer device comes with instructions for use and care and cleaning that you should read carefully.
Caring for your inhaler
It is important that you follow these instructions carefully and that you clean your inhaler weekly. To clean your inhaler:
- Remove the mouthpiece cover.
- Do not remove the metal canister from the plastic casing.
- Clean the inside and outside of the mouthpiece and the plastic casing with a dry and clean cloth or a paper tissue.
- Replace the mouthpiece cover.
- Do not put the metal canister in water.
If you use more Flutiform than you should
It is important that you take your doses as directed by your doctor or pharmacist. Do not increase or decrease your dose without medical advice.
If you use more Flutiform than you should, contact your doctor or pharmacist immediately for advice. You may experience severe chest pain (angina), high or low blood pressure, headache, muscle cramps, difficulty sleeping, nervousness, dry mouth, loss of appetite, agitation, fits or convulsions. You may feel unsteady, dizzy, weak, tired, sick or generally unwell. You may also notice changes in your heart rate and you may have low blood potassium levels or high blood sugar levels. You may also experience symptoms such as abdominal pain, feeling unwell, weight loss, decreased level of consciousness (which can cause drowsiness or confusion) or low blood sugar levels.
If you have taken more doses than prescribed over a long period of time, you should talk to your doctor or pharmacist for advice on what to do. This is because high doses may reduce the amount of steroid hormones produced normally by the adrenal glands (see section 4 "Possible side effects").
If you forget to use Flutiform
If you forget to take a dose, take it as soon as you remember. However, if it is almost time for your next dose, skip the missed dose. Do not take a double dose to make up for a forgotten dose.
If you stop using Flutiform
It is very important that you take this inhaler every day, as directed by your doctor, even if you feel well, as it will help to control your asthma. If you want to stop using your inhaler, talk to your doctor first; he will advise you how to do it, usually by gradually reducing the dose, so as not to trigger an asthma attack.
4. Possible Adverse Effects
Like all medicines, this inhaler can cause adverse effects, although not all people suffer from them. Your doctor will prescribe the lowest necessary dose to control your asthma, which may reduce the likelihood of adverse effects.
All medicines can cause allergic reactions, although severe allergic reactions are rare. Inform your doctor immediately if your eyelids, face, throat, tongue, or lips suddenly swell; if you have a skin rash or feel itching, especially if it occurs all over your body; if you have symptoms such as dizziness, feeling faint, or changes in your breathing, such as an increase in wheezing or difficulty breathing.
As with other inhalers, your breathing may worsen immediately after using the inhaler. You may notice an increase in wheezing and difficulty breathing. If this happens, stop using Flutiform and use your 'rescue' inhaler with rapid action. Contact your doctor immediately. He will perform an evaluation and may initiate a different type of treatment. You must carry your 'rescue' inhaler with you at all times.
Uncommon Adverse Effects (may affect up to 1 in 100 people)
- Asthma worsening.
- Headache.
- Tremors.
- Irregular heartbeat or palpitations.
- Dizziness.
- Difficulty sleeping.
- Voice changes / hoarseness.
- Dry mouth, sores, or throat irritation.
- Rash.
Rare Adverse Effects (may affect up to 1 in 1,000 people)
- Increased blood sugar levels. If you are diabetic, you may need to check your blood sugar more often and adjust your usual diabetes treatment. Your doctor may need to monitor you more closely.
- Candidiasis or other fungal infections in the mouth and throat.
- Sinusitis (inflammation of the paranasal sinuses).
- Fast heartbeat.
- Chest pain associated with heart disease.
- Muscle spasms.
- Cough or difficulty breathing.
- Diarrhea.
- Indigestion.
- Changes in taste.
- A feeling of dizziness or lightheadedness.
- Abnormal dreams.
- Agitation.
- Itching of the skin.
- High blood pressure.
- A feeling of unusual weakness.
- Swelling of hands, ankles, or feet.
Frequency Not Known: cannot be estimated from the available data
- Blurred vision.
- Sleep problems, depression, or feelings of worry, aggression, anxiety, restlessness, nervousness, overexcitement, or irritability. These effects are more likely to occur in children.
The following adverse effects have been associated with formoterol fumarate, but have not been reported during clinical trials with this inhaler:
- Low potassium levels, which can cause muscle weakness, muscle spasms, or changes in heart rhythm.
- An abnormal electrocardiogram that may potentially reveal an abnormal heart rhythm (prolongation of the QTc interval).
- High levels of lactic acid in the blood.
- Feeling unwell.
- Muscle pain.
Inhaled steroids may affect the normal production of steroid hormones in your body, especially if you use high doses for a long time. The effects include:
- Changes in bone mineral density (thinning of the bones).
- Cataracts (opacity of the eye lens).
- Glaucoma (increased eye pressure).
- Bruising or thinning of the skin.
- Increased likelihood of infection.
- Slowing of growth in children and adolescents.
- Round face (moon face).
- An effect on the adrenal gland (a small gland above the kidney), which can cause symptoms such as weakness, fatigue, difficulty coping with stress, abdominal pain, loss of appetite, weight loss, headache, dizziness, very low blood pressure, diarrhea, feeling unwell, or convulsions.
These effects are much less likely to occur with inhaled steroids than with steroid tablets.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Flutiform
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the label, the aluminum bag, and the cardboard box, after CAD. The expiration date is the last day of the month indicated. CAD: 08-2020 means that you should not use the inhaler after the last day of that month, i.e., August 2020.
Do not store at a temperature above 25°C. Do not refrigerate or freeze. If the inhaler is exposed to freezing conditions, let it come to room temperature for 30 minutes and then load it before use (see section 3 "How to use Flutiform"). Do not use the inhaler if it has been removed from the aluminum bag for more than 3 months, or if the dose indicator marks '0'.
Do not expose to temperatures above 50°C. The aerosol cartridge contains a pressurized liquid, so it should not be pierced, broken, or burned, even if it appears to be empty.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE collection point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Flutiform
- The active ingredients are fluticasone propionate and formoterol fumarate dihydrate. Each inhalation (puff) contains 250 micrograms of fluticasone propionate and 10 micrograms of formoterol fumarate dihydrate.
- The other ingredients (excipients) are:
- Sodium cromoglicate
- Ethanol
- Apaflurane HFA 227 (propellant)
This medicine contains fluorinated greenhouse gases.
Each inhaler contains 11.2 grams of fluorinated greenhouse gas HFA227, which corresponds to 0.036 tons of CO2 equivalent (global warming potential GWP = 3220).
Appearance of Flutiform and Package Contents
This inhaler is a small aerosol cartridge that contains a white to off-white liquid suspension equipped with a metering valve. The cartridge is inserted into a gray and white plastic dispenser (applicator) with a light gray cover for the mouthpiece. Each inhaler contains 120 doses (inhalations). There is one inhaler in each package. The multipack is 3 x 1 inhaler (120 inhalations).
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Mundipharma Pharmaceuticals, S.L.
Bahía de Pollensa, 11
28042 Madrid
Spain
Phone: 91 3821870
Manufacturer(s)
Mundipharma DC B.V.
Leusderend 16
3832 RC Leusden
Netherlands
This medicine is authorized in the Member States of the European Economic Area under the following names:
Flutiform:
Austria
Belgium
Bulgaria
Cyprus
Croatia
Czech Republic
Denmark
Finland
France
Germany
Hungary
Ireland
Iceland
Luxembourg
Netherlands
Norway
Poland
Portugal
Romania
Slovakia
Slovenia
Spain
Sweden
Flutiformo
Italy
Date of the Last Revision of this Leaflet:02/2025
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price66.78 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUTIFORM 250 micrograms / 10 micrograms INHALATION SUSPENSION in pressurized containerDosage form: PULMONARY INHALATION, 125 micrograms/5 micrograms/doseActive substance: formoterol and fluticasoneManufacturer: Mundipharma Pharmaceuticals S.L.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/5 micrograms/doseActive substance: formoterol and fluticasoneManufacturer: Mundipharma Pharmaceuticals S.L.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/250 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription required
Online doctors for FLUTIFORM 250 micrograms / 10 micrograms INHALATION SUSPENSION in pressurized container
Discuss questions about FLUTIFORM 250 micrograms / 10 micrograms INHALATION SUSPENSION in pressurized container, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















