
FLUMIL 100 mg/mL Injectable Solution


How to use FLUMIL 100 mg/mL Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information Leaflet
Flumil 100 mg/ml Solution for Injection
Acetylcysteine
Read this leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What Flumil 100 mg/ml is and what it is used for
- What you need to know before taking Flumil 100 mg/ml
- How to take Flumil 100 mg/ml
- Possible side effects
5. Storage of Flumil 100 mg/ml
- Package Contents and Additional Information
1. What Flumil 100 mg/ml is and what it is used for
Flumil 100 mg/ml contains the active ingredient acetylcysteine, which belongs to a group of medicines called mucolytics that reduce the viscosity of mucus, making it more fluid and easier to eliminate from the respiratory tract.
Flumil 100 mg/ml is indicated to facilitate the elimination of excess mucus and phlegm in adults and children from 2 years of age, in respiratory processes with bronchial hypersecretion such as: acute and chronic bronchitis, chronic obstructive pulmonary disease (COPD), emphysema, pulmonary complications of cystic fibrosis, facilitation of maneuvers in anesthesia for bronchoscopies, bronchographies, and bronchoaspiration, bronchiectasis, obstructive and infectious complications due to tracheotomy and bronchopulmonary due to surgical intervention.
2. What you need to know before taking Flumil 100 mg/ml
Do not use Flumil 100 mg/ml:
- If you are allergic to the active ingredient or any of the other components of this medicine (listed in section 6).
- Do not administer to children under 2 years of age.
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Flumil 100 mg/ml.
If you have asthma or a severe respiratory disease, you should consult your doctor before taking this medicine, as it may cause respiratory difficulties (bronchospasm).
Patients with bronchial asthma should be closely monitored during treatment. If bronchospasm occurs, acetylcysteine should be discontinued immediately and appropriate treatment initiated. This bronchoconstrictor effect can be prevented by prior administration of a bronchodilator.
The possible sulfurous odor (like rotten eggs) of the medicine is characteristic of the active ingredient, but it does not indicate that its characteristics have been altered.
Caution is recommended when using the product in patients with peptic ulcer or history of peptic ulcer, especially in cases of concomitant administration of other medicines with a known irritating effect on the gastric mucosa.
During the first days of treatment, you may observe an increase in mucus and phlegm, which will decrease throughout the treatment. If you see that you are not able to expectorate effectively, postural drainage and bronchoaspiration should be performed.
Intravenous administration will be carried out under strict medical supervision. It is more likely that adverse reactions will appear after intravenous perfusion if the drug is administered too quickly or in excess. Therefore, it is recommended to strictly follow the instructions that appear in section 3. How to use Flumil 100 mg/ml.
Acetylcysteine is not compatible with rubber and certain metals, especially iron, nickel, and copper. Contact with materials containing them should be avoided.
It should be administered with caution in long-term treatment in patients with histamine intolerance.
Children and Adolescents
In children and adolescents, the same precautions and warnings apply.
It is contraindicated in children under 2 years of age.
Other Medicines and Flumil 100 mg/ml
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
In case you need simultaneous treatment with nitroglycerin, you should monitor the appearance of hypotension (low blood pressure), which can be severe, and may cause headache.
The simultaneous use of carbamazepine, a drug used to combat epilepsy attacks, may increase the risk of attacks.
Do not administer concomitantly with antitussive medicines (for cough) or with those that decrease bronchial secretions (such as antihistamines and anticholinergics), as it may lead to accumulation of bronchial secretions.
Administration of antibiotics is recommended separately.
It is not recommended to dissolve Flumil 100 mg/ml with other medicines.
Using Flumil 100 mg/ml with Food and Drinks
Taking food and drinks does not affect the efficacy of this medicine.
Pregnancy, Breastfeeding, and Fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Acetylcysteine crosses the placenta. Therefore, the use of acetylcysteine during pregnancy is not recommended.
It is unknown whether acetylcysteine and its metabolites are excreted in breast milk. Its use during breastfeeding should be avoided.
No data are available on the effect of acetylcysteine on human fertility. Animal studies do not indicate harmful effects on human fertility at recommended doses.
Driving and Using Machines
There is no evidence of effects on the ability to drive and use machines.
Flumil 100 mg/ml contains Sodium
This medicine contains 43 mg of sodium (main component of table salt) in each 3 ml ampoule. This is equivalent to 2.15% of the maximum recommended daily sodium intake for an adult.
Interference with Analytical Tests
Acetylcysteine may interfere with the colorimetric method for determining salicylates.
Acetylcysteine may interfere with the ketone test in urine.
3. How to use Flumil 100 mg/ml
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is:
Local Administration
Inhalation by nebulization:
Adults and children from 12 years of age: one 300 mg ampoule one or two times a day for 5 to 10 days.
Children between 2 and 12 years of age: up to one 300 mg ampoule one or two times a day for 5 to 10 days in children who cooperate.
Endotracheobronchial route:
Adults and children from 12 years of age: one 300 mg ampoule (60 drops) one or two times a day for 5 to 10 days.
Children between 2 and 12 years of age: up to one 300 mg ampoule (60 drops) one or two times a day for 5 to 10 days.
Parenteral Administration
Flumil 100 mg/ml can be administered in bronchial conditions when local treatment is impossible or difficult, or when the doctor prefers systemic treatment (lack of patient cooperation, bed rest, closed circuit breathing, etc.).
Intramuscular route:
Adults and children from 12 years of age: one 300 mg ampoule one or two times a day administered by deep injection.
Children between 2 and 12 years of age: 150 mg (half a 3 ml ampoule) one or two times a day administered by deep injection.
Intravenous route:
The administration of acetylcysteine by intravenous route is carried out under strict medical supervision.
The medicine should be administered by slow perfusion in saline solution or 5% glucose solution.
Adults and children from 12 years of age: one 300 mg ampoule one or two times a day.
Children between 2 and 12 years of age: 150 mg (half a 3 ml ampoule) one or two times a day.
Opening the ampoule:
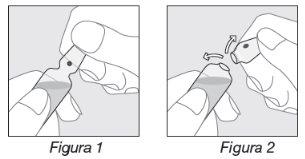
- Hold the ampoule as indicated in Figure 1;
- Place your thumb on the colored point and exert pressure backwards as indicated in Figure 2.
Duration of Treatment
The duration of treatment should be established according to clinical evolution. The average duration is 5-10 days. The high general and local tolerability of Flumil 100 mg/ml allows for prolonged treatments in certain cases.
If you use more Flumil 100 mg/ml than you should
If you have used more Flumil 100 mg/ml than you should, you may notice symptoms similar to those described in section 4, Possible side effects, although more intense. In case of overdose or accidental massive administration, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
The following side effects may occur, although their frequency cannot be established from the available information:
Local use:
Allergic reactions (hypersensitivity), constriction of the bronchi and difficulty breathing (bronchospasm), increased nasal secretion (rhinorrhea), mouth sores (stomatitis), vomiting, nausea, urticaria, rash, or itching.
Parenteral use:
Allergic reactions (hypersensitivity) of varying degrees, which can lead to anaphylactic shock, increased heart rate (tachycardia), constriction of the bronchi and difficulty breathing (bronchospasm, dyspnea), vomiting, nausea, facial swelling (angioedema), urticaria, flushing, rash, itching, facial edema, decreased blood pressure, decreased blood coagulation (increased prothrombin time, decreased platelet aggregation).
In very rare cases, severe skin reactions (Stevens-Johnson syndrome and Lyell syndrome) may appear, although in most cases, at least one other suspect drug could be identified as triggering the syndrome.
In case of any alteration in the skin or mucous membranes, the administration of acetylcysteine should be discontinued immediately. The specialist doctor will determine the treatment to follow.
Reporting of Side Effects
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect that does not appear in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Flumil 100 mg/ml
Keep this medicine out of the sight and reach of children.
No special storage conditions are required in its original packaging.
Do not use this medicine after the expiration date that appears on the packaging after the abbreviation CAD. The expiration date is the last day of the month indicated.
Local Administration
It is recommended to open the ampoule at the time of use. Opened ampoules can only be used for local use and should be stored in the refrigerator for a maximum of 24 hours.
Parenteral Administration
Once opened, use immediately. If not used immediately, the times and conditions of use are the responsibility of the user.
The solution, once diluted for use (in 5% glucose solution or 0.9% sodium chloride solution), remains stable for a period of 24 hours at 25°C.
Discard after use.
Medicines should not be thrown away via wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Flumil 100 mg/ml
The active ingredient is acetylcysteine. Each 3 ml ampoule contains 300 mg of acetylcysteine.
The other components (excipients) are: disodium edetate, sodium hydroxide (E524) (for pH adjustment), and water for injectable preparations.
Appearance of the Product and Package Contents
Topaz glass ampoules with a breaking point, containing 3 ml of a clear and colorless solution.
Each package contains 5 ampoules.
Marketing Authorization Holder and Manufacturer
Zambon S.A.U.
Maresme, 5. Pol. Can Bernades-Subirà
08130 Sta. Perpètua de Mogoda (Barcelona)
Spain
Manufacturer
Zambon S.p.A.
Via della Chimica, 9
36100 Vicenza
Italy
or
Biologici Italia Laboratories S.r.l.
Via Filippo Serpero, 2
20060 Masate (MI)
Italy
Date of the Last Revision of this Leaflet: May 2025
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUMIL 100 mg/mL Injectable SolutionDosage form: EFFERVESCENT TABLET, 600 mgActive substance: acetylcysteineManufacturer: Kern Pharma S.L.Prescription not requiredDosage form: EFFERVESCENT TABLET, 600 mgActive substance: acetylcysteineManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: EFFERVESCENT TABLET, 200 mgActive substance: acetylcysteineManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for FLUMIL 100 mg/mL Injectable Solution
Discuss questions about FLUMIL 100 mg/mL Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















