
FLUENZ NASAL SPRAY SUSPENSION

How to use FLUENZ NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fluenz Nasal Suspension for Inhalation
Vaccine against influenza (live, nasal)
Read all of this leaflet carefully before the vaccine is administered because it contains important information for you or your child.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, nurse or pharmacist.
- This vaccine has been prescribed only for you or your child, do not pass it on to others.
- If you experience any side effects, talk to your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet:
- What is Fluenz and what is it used for
- What you need to know before Fluenz is administered
- How Fluenz is administered
- Possible side effects
- Storage of Fluenz
- Contents of the pack and further information
1. What is Fluenz and what is it used for
Fluenz is a vaccine to prevent influenza. It is used in children and adolescents from 2 years to less than 18 years of age. Fluenz will help to protect you against the strains of virus contained in the vaccine and other closely related strains.
How Fluenz works
When the vaccine is administered to a person, the immune system (the body's natural defense system) produces its own protection against the influenza virus. None of the components of the vaccine can cause influenza.
The Fluenz vaccine viruses are grown in chicken eggs. Each year, the vaccine protects against three strains of influenza, following the annual recommendations of the World Health Organization.
2. What you need to know before Fluenz is administered
Fluenz will not be administered:
- if you are allergicto the active substances, gentamicin (a residual trace from the manufacturing process), or to any of the other components of this vaccine listed in section 6.
- if you have ever had a severe allergic reaction to eggs or egg proteins. For signs of allergic reactions, see section 4.
- if you have a blood disorderor cancerthat affects the immune system.
- if your doctor has told youthat you have a weakened immune systemdue to a disease, medication, or other treatment (such as acute and chronic leukemia, lymphoma, symptomatic HIV infection, cellular immunodeficiencies, and high doses of corticosteroids).
- if you are already takingacetylsalicylic acid(a substance found in many medications used to relieve pain and reduce fever). This is due to the risk of a very rare but serious disease (Reye's syndrome).
If any of these conditions apply, tell your doctor, nurse or pharmacist.
Warnings and precautions
Your doctor or nurse will ensure that you have adequate medical treatment and supervision in case a rare anaphylactic reaction (a very severe allergic reaction with symptoms such as difficulty breathing, dizziness, weak and rapid pulse, and skin rash) occurs after administration.
Tell your doctor, nurse or pharmacist before vaccination:
- if you have severe asthmaor are currently wheezing.
- if you have a severe acute illnessassociated with fever or infection, or if you have nasal obstruction. Your doctor may decide to delay vaccination until the fever or nasal obstruction has resolved.
- if you are in close contact with someone who has a severely weakened immune system(e.g., a bone marrow transplant patient who requires isolation).
- if you have a congenital defectthat affects the bones and tissues of the skull and face, which has not been surgically corrected.
If any of these conditions apply, tell your doctor, nurse or pharmacist before vaccination. They will decide if Fluenz is suitable for you.
Children under 2 years of age
Children under 2 years of age must not receive this vaccine due to the risk of side effects.
Other medicines, other vaccines, and Fluenz
Tell your doctor, nurse or pharmacist if the person to be vaccinated is using, has recently used, or might use any other medicines, including those that do not require a prescription.
- Do not administeracetylsalicylic acid(a substance found in many medications used to relieve pain and reduce fever) to childrenfor 4 weeks after vaccination with Fluenz unless your doctor, nurse or pharmacist tells you otherwise. This is due to the risk of Reye's syndrome, a very rare but serious disease that can affect the brain and liver.
- It is recommended not to administer Fluenzat the same time as antiviral medicationssuch as oseltamivir and zanamivir. This is because the vaccine may lose its effectiveness.
Your doctor, nurse or pharmacist will decide if Fluenz can be administered at the same time as other vaccines.
Pregnancy and breastfeeding
- If you are pregnant, think you may be pregnant, plan to become pregnant, or are breastfeeding, consult your doctor, nurse or pharmacistbefore using this vaccine. Fluenz is not recommendedfor pregnant or breastfeeding women.
Driving and using machines
- Fluenz has no or negligible influence on the ability to drive and use machines.
3. How Fluenz is administered
Fluenz should only be used for nasal inhalation.
Fluenz must not be injected.
Fluenz will be administered as a spray into each nostril. You can breathe normally while Fluenz is being administered. You do not need to inhale or sniff actively.
Dosage
The recommended dosefor children and adolescents is 0.2 ml of Fluenz, administered as 0.1 ml into each nostril. Children from 2 to 8 years of age who have not been vaccinated against influenza beforewill receive a second follow-up dose at least 4 weeks after the first dose. Follow the instructions of your doctor, nurse or pharmacist regarding whether your child should attend for the second dose and when.
If you have any further questions on the use of this medicine, ask your doctor, nurse or pharmacist.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Ask your doctor, nurse or pharmacist for more information on the possible side effects of Fluenz.
Some side effects may be serious.
Very rare(may affect up to 1 in 10,000 people)
- severe allergic reactions: signs of allergic reaction may include difficulty breathing and swelling of the face or tongue.
Tell your doctor immediately or seek urgent medical attention if you notice any of these symptoms.
Other possible side effects of Fluenz
Very common(may affect more than 1 in 10 people)
- stuffy or runny nose
- loss of appetite
- feeling unwell
Common(may affect up to 1 in 10 people)
- fever
- muscle pain
- headache
Uncommon(may affect up to 1 in 100 people)
- skin rash
- nasal bleeding
- allergic reactions
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fluenz
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date stated on the label of the applicator and on the carton after the letters EXP/CAD.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the nasal applicator in the outer packaging to protect it from light.
Before use, the vaccine can be removed from the refrigerator once for a maximum period of 12 hours at a temperature of up to 25°C. If it is not used within this 12-hour period, the vaccine must be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Fluenz
The active substances are:
Influenza virus reassorted* (live attenuated) of the following three strains**:
Strain similar to A/Victoria/4897/2022 (H1N1)pdm09
(A/Norway/31694/2022, MEDI 369815) 10^7.0±0.5 FFU***
Strain similar to A/Croatia/10136RV/2023 (H3N2)
(A/Perth/722/2024, MEDI 392611) 10^7.0±0.5 FFU***
Strain similar to B/Austria/1359417/2021
(B/Austria/1359417/2021, MEDI 355292) 10^7.0±0.5 FFU***
.......................................................................................................per 0.2 ml dose
- Multiplied in fertilized chicken eggs from healthy chicken farms.
** Produced in VERO cells by reverse genetic technology. This product contains genetically modified organisms (GMOs).
*** Fluorescent Focus Units
This vaccine complies with the recommendation of the WHO (World Health Organization, Northern Hemisphere) and the EU decision for the 2025/2026 season.
The vaccine may contain traces of the following substances: egg proteins (e.g., ovalbumin) and gentamicin. The other components are sucrose, potassium hydrogen phosphate, potassium dihydrogen phosphate, gelatin, arginine hydrochloride, monosodium glutamate monohydrate, and water for injection.
Appearance of Fluenz and contents of the pack
This vaccine is presented as a suspension for nasal inhalation (0.2 ml) in a single-use nasal applicator (Type 1 glass) in a pack size of 1 and 10 nasal applicators. Not all pack sizes may be marketed in your country.
The suspension is colorless to pale yellow, transparent to slightly turbid. It may contain small white particles.
Marketing authorization holder
AstraZeneca AB,
SE-151 85
Södertälje
Sweden
Manufacturer
AstraZeneca Nijmegen B.V.,
Lagelandseweg 78
Nijmegen, 6545CG
Netherlands
You can request more information about this medicinal product from the local representative of the marketing authorization holder:
België/Belgique/Belgien AstraZeneca S.A./N.V. Tel: +32 2 370 48 11 | Lietuva UAB AstraZeneca Lietuva Tel: +370 5 2660550 |
| Luxembourg/Luxemburg AstraZeneca S.A./N.V. Tél/Tel: +32 2 370 48 11 |
Ceská republika AstraZeneca Czech Republic s.r.o. Tel: +420 222 807 111 | Magyarország AstraZeneca Kft. Tel.: +36 1 883 6500 |
Danmark AstraZeneca A/S Tlf: +45 43 66 64 62 | Malta Associated Drug Co. Ltd Tel: +356 2277 8000 |
Deutschland AstraZeneca GmbH Tel: +49 40 809034100 | Nederland AstraZeneca BV Tel: +31 85 808 9900 |
Eesti AstraZeneca Tel: +372 6549 600 | Norge AstraZeneca AS Tlf: +47 21 00 64 00 |
Ελλάδα AstraZeneca A.E. Τηλ: +30 210 6871500 | Österreich AstraZeneca Österreich GmbH Tel: +43 1 711 31 0 |
España AstraZeneca Farmacéutica Spain, S.A. Tel: +34 91 301 91 00 | Polska AstraZeneca Pharma Poland Sp. z o.o. Tel.: +48 22 245 73 00 |
France AstraZeneca Tél: +33 1 41 29 40 00 | Portugal AstraZeneca Produtos Farmacêuticos, Lda. Tel: +351 21 434 61 00 |
Hrvatska AstraZeneca d.o.o. Tel: +385 1 4628 000 | România AstraZeneca Pharma SRL Tel: +40 21 317 60 41 |
Ireland AstraZeneca Pharmaceuticals (Ireland) DAC Tel: +353 1609 7100 | Slovenija AstraZeneca UK Limited Tel: +386 1 51 35 600 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika AstraZeneca AB, o.z. Tel: +421 2 5737 7777 |
Italia AstraZeneca S.p.A. Tel: +39 02 00704500 | Suomi/Finland AstraZeneca Oy Puh/Tel: +358 10 23 010 |
Κύπρος Αλκτωρ Φαρμακευτική Λτδ Τηλ: +357 22490305 | Sverige AstraZeneca AB Tel: +46 8 553 26 000 |
Latvija SIA AstraZeneca Latvija Tel: +371 67377100 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website https://www.ema.europa.eu.
-----
Instructions for healthcare professionals
This information is intended only for healthcare professionals:
Fluenz is for nasal use only.
- Do not use with a needle.Do not inject.
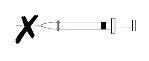
- Do not use Fluenz if the expiry date has expired or if the applicator is damaged, for example, if the plunger is loose or displaced from the nasal applicator or if there is any sign of loss of content.
- Check the appearance of the vaccine before administration. The suspension should be colorless to pale yellow, transparent to opalescent. It may contain small white particles.
- Fluenz is administered as a divided dose into both nostrils as described below (see section 3).
- After administering half of the dose into one nostril, administer the other half of the dose into the other nostril immediately or shortly after.
- The patient can breathe normally while the vaccine is being administered; there is no need to inhale or sniff actively.
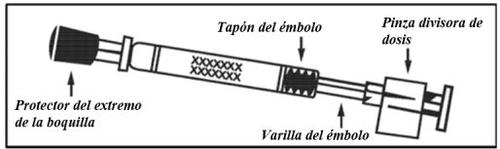
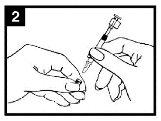
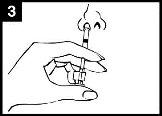
Check the expiry date The product must not be used after the date stated on the label of the applicator. | Prepare the applicator Remove the protector from the end of the nozzle. Do not remove the dose divider from the other end of the applicator. | Place the applicator With the patient in an upright position, place the end inside the nostril to ensure that Fluenz is administered into the nose. |
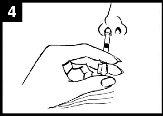

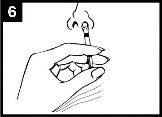
Press the plunger With a single movement, press the plunger as quickly as possibleuntil the dose divider prevents further movement. | Remove the dose divider To administer into the other nostril, pinch and remove the dose divider from the plunger. | Spray into the other nostril Place the end immediately inside the other nostriland, with a single movement, press the plunger as quickly as possibleto administer the rest of the vaccine. |
See section 5for information on storage and disposal
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUENZ NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 7.0 ± 0.5 LOG10 FFUActive substance: influenza, live attenuatedManufacturer: Astrazeneca AbPrescription requiredDosage form: INJECTABLE, 3.75 microgramsActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 0.5 mlActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for FLUENZ NASAL SPRAY SUSPENSION
Discuss questions about FLUENZ NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















