FLUARIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE

How to use FLUARIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet:information for the user
Fluarix injectable suspension in pre-filled syringe
Influenza vaccine (trivalent, inactivated, split virion)
This package leaflet has been written to be read by the person receiving the vaccine. However, it may be read by someone else.
Read all of this leaflet carefully before you start receiving this vaccine,because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed for you. Do not pass it on to others.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Fluarix and what is it used for
- What you need to know before you receive Fluarix
- How Fluarix is administered
- Possible side effects
- Storage of Fluarix
- Contents of the pack and other information
1. What is Fluarix and what is it used for
Fluarix is a vaccine to prevent influenza in adults and children from 6 months of age.
Influenza is a disease of the upper respiratory tract and lungs caused by an influenza virus infection. The most common symptoms of influenza are: high fever, sore throat, cough, general aches, headache, weakness, and fatigue. Complications can occur, especially in very young, very old people, and those with low immunity to infections.
How does Fluarix work?
- Fluarix contains killed viruses that cannot cause influenza.
- Fluarix stimulates the body's immune system to produce antibodies against the specific viruses the vaccine is designed to protect against. This will help prevent the disease.
- The vaccine is targeted against influenza viruses according to official recommendations.
- As with all vaccines, it is possible that Fluarix may not completely protect all vaccinated individuals.
2. What you need to know before you receive Fluarix
Do not receiveFluarix if:
- you are allergic to the active substances or any of the other components of this vaccine (listed in section 6) or to any component that may be present in very small amounts, such as ovalbumin (an egg protein), hydrocortisone, gentamicin sulfate, formaldehyde, and sodium deoxycholate.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before receiving Fluarix if:
- you have a severe infection with high fever. If this happens, vaccination may need to be postponed until you feel better. A minor infection, such as a cold, should not be a problem, but talk to your doctor, pharmacist, or nurse first.
- you have a bleeding problem or bruise easily.
Fainting (syncope) can occur following, or even before, any injection, especially in adolescents. Tell your doctor, pharmacist, or nurse if you have fainted with a previous injection.
People with weakened immune systems, for example due to HIV infection or due to medicines that suppress the immune system, may not get the full benefit of Fluarix.
If any of the above applies to you (or you are not sure), talk to your doctor, pharmacist, or nurse before receiving Fluarix.
Other medicines and vaccines and Fluarix
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines or have recently received any other vaccine.
If Fluarix is to be given at the same time as other vaccines, a different injection site should be used for each type of vaccine.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before receiving this vaccine.
Driving and using machines
Some of the effects mentioned below in section 4 “Possible side effects” (e.g. fatigue or dizziness) may temporarily affect your ability to drive or use machines. Do not drive or use machines or tools if you do not feel well.
Fluarix contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per dose; this is essentially “sodium-free”.
Fluarix contains potassium
This medicine contains less than 39 mg (1 mmol) of potassium per dose, and is therefore considered to be “potassium-free”.
Fluarix contains polysorbate 80
This medicine does not contain more than 0.415 mg of polysorbate 80 per dose. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergy to this substance.
3. How Fluarix is administered
Fluarix is given as a single injection of 0.5 ml into a muscle.
Use in children
Children under 9 years of age who have not been previously vaccinated against influenza will receive a second injection at least one month after the first. Make sure your child completes the vaccination course. This will maximize the protection provided by Fluarix.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Talk to your doctor, pharmacist, or nurse if you want more information on the possible side effects of Fluarix.
Side effects reported during general use of Fluarix:
Serious side effects
Tell your doctor immediately if you notice any of the following serious side effects; you may need urgent medical treatment.
Rare:these may occur in up to 1 in 1,000 doses of the vaccine
- Severe allergic reactions (anaphylactic reactions). These can be recognized by:
- itchy rash on hands and feet
- swelling of the eyes and face
- difficulty breathing or swallowing
- sudden drop in blood pressure and loss of consciousness.
These reactions usually occur within 15 minutes after vaccination. However, if you experience any of these symptoms, contact a doctor urgently.
Other side effects
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects.
Rare:these may occur in up to 1 in 1,000 doses of the vaccine
- Nerve inflammation (neuritis), brain and spinal cord inflammation (encephalomyelitis), temporary nerve inflammation causing pain, weakness, and paralysis, called Guillain-Barré syndrome.
- Skin reactions that can spread all over the body, including itching (pruritus, urticaria) and skin redness (erythema), rash.
- Temporary inflammation of the glands in the neck, armpits, or groin (transient lymphadenopathy)
- Flu-like symptoms, general feeling of being unwell.
Tell your doctor, pharmacist, or nurse if you notice any of the above side effects.
Side effects that occurred during clinical trials with Fluarix:
Side effects that occurred in children between 6 months and less than 6 years of age
Very common:these may occur in more than 1 in 10 doses of the vaccine
- Lack of appetite.
- Irritability.
- Sleepiness.
- Pain at the injection site.
- Redness at the injection site.
- Swelling at the injection site.
Common:these may occur in up to 1 in 10 doses of the vaccine
- Nausea.
- Vomiting.
- Diarrhea.
- Stomach pain.
- Fever.
Side effects that occurred in children between 6 years and less than 18 years of age
Very common:these may occur in more than 1 in 10 doses of the vaccine
- Headache.
- Muscle pain.
- Fatigue.
- Pain at the injection site.
- Redness at the injection site.
- Swelling at the injection site.
Common:these may occur in up to 1 in 10 doses of the vaccine
- Nausea.
- Vomiting.
- Diarrhea.
- Stomach pain.
- Joint pain.
- Chills.
- Fever.
Side effects that occurred in adults from 18 years of age
Very common:these may occur in more than 1 in 10 doses of the vaccine
- Pain at the injection site.
- Fatigue.
- Headache.
- Muscle pain.
Common:these may occur in up to 1 in 10 doses of the vaccine
- Redness, swelling, or a hard lump at the injection site.
- Chills.
- Sweating.
- Joint pain.
- Nausea.
- Vomiting.
- Diarrhea.
- Stomach pain.
Uncommon:these may occur in up to 1 in 100 doses of the vaccine
- Fever.
- Dizziness.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines and Healthcare Products Agency (AEMPS) website (http://www.aemps.gob.es/). By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fluarix
- Keep this vaccine out of the sight and reach of children.
- Do not use this vaccine after the expiry date which is stated on the label and carton, after EXP. The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze.
- Store in the outer carton to protect from light.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Fluarix
The active substance is influenza virus (inactivated, split virion) of the following strains*:
Strain similar to A/Victoria/4897/2022 (H1N1)pdm09:
(IVR-238) derived from A/Victoria/4897/202215 micrograms of HA**
Strain similar to A/Croatia/10136RV/2023 (H3N2):
(X-425A) derived from A/Croatia/10136RV/202315 micrograms of HA**
Strain similar to B/Austria/1359417/2021:
(BVR-26) derived from B/Austria/1359417/202115 micrograms of HA**
per 0.5 ml dose
- propagated in embryonated hens' eggs from healthy chicken flocks
** hemagglutinin
This vaccine complies with the World Health Organization (WHO) recommendation for the Northern Hemisphere and with the European Union recommendation for the 2025/2026 season.
The other ingredients are: sodium chloride, disodium phosphate dodecahydrate, potassium dihydrogen phosphate, potassium chloride, magnesium chloride hexahydrate, α-tocopherol hydrogen succinate, polysorbate 80, octoxinol 10, and water for injections
Appearance and pack contents
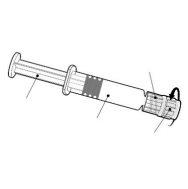
- Fluarix is an injectable suspension in a pre-filled syringe.
- Fluarix is a colorless, slightly opalescent liquid.
- Fluarix is available in a pre-filled syringe with or without a separate needle; pack sizes of 1 and 10.
- Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel.: +34 900 202 700
Manufacturer
GlaxoSmithKline Biologicals
Branch of SmithKline Beecham Pharma GmbH & Co. KG
Zirkusstrasse 40
01069 Dresden
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Member State | Name |
Austria | Fluarix Trivalent |
Belgium, Luxembourg | Alpharix |
Spain, Finland, France, Italy, Norway, Netherlands, Poland, Portugal, Sweden | Fluarix |
Germany, Greece | Influsplit |
Date of last revision of this leaflet:07/2025
Other sources of information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) (http://www.aemps.gob.es/).
-----------------------------------------------------
This information is intended only for healthcare professionals:
Vaccines should be inspected visually for any foreign particles and/or changes in physical appearance before administration.
Before use, the vaccine should be shaken well to obtain a colorless, slightly opalescent liquid. Discard if the contents appear otherwise. Inject the entire contents of the syringe.
Instructions for the pre-filled syringe
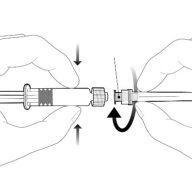
| Hold the syringe by the body, not by the plunger. Unscrew the syringe cap by twisting it counterclockwise. |
| To insert the needle, attach the base to the luer-lock adapter and twist it a quarter turn clockwise until it clicks. Do not pull the plunger out of the syringe body. If this happens, do not administer the vaccine. |
Disposal of waste
Disposal of unused medicinal products and all materials that have been in contact with them should be done in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUARIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 3.75 microgramsActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 0.5 mlActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 60 micrograms of HAActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for FLUARIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about FLUARIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions