EFLUELDA SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE

How to use EFLUELDA SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Efluelda injectable suspension in a pre-filled syringe
Influenza vaccine (split virion, inactivated) 60 micrograms HA/strain
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you are vaccinated because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Efluelda and what is it used for
- What you need to know before you use Efluelda
- How to use Efluelda
- Possible side effects
- Storage of Efluelda
- Contents of the pack and other information
1. What is Efluelda and what is it used for
Efluelda is a vaccine. This vaccine helps to protect people aged 60 years and older against flu. The use of Efluelda should be based on official recommendations for flu vaccination.
When you receive Efluelda, your immune system (the body's natural defenses) will produce its own protection (antibodies) against the disease. None of the components of the vaccine can cause flu.
Flu is a contagious respiratory illness caused by the flu virus, which can result in mild to severe illness, and serious complications such as pneumonia, which can lead to hospitalization or even death. Flu is an illness that can spread quickly and is caused by different types of strains that can change every year. Due to this potential change in circulating strains annually, as well as the duration of the protection provided by the vaccine, annual vaccination is recommended. The greatest risk of getting flu is during the cold months between October and March. If you were not vaccinated in the fall, it is still a good idea to get vaccinated until spring, since you are at risk of getting flu until then. Your doctor will be able to recommend the best date for vaccination.
Efluelda is designed to protect you against the three strains of the virus contained in the vaccine approximately 2 to 3 weeks after injection. Additionally, if you are exposed to flu immediately before or after vaccination, you may still develop the illness, since the incubation period of flu is a few days.
The vaccine will not protect you against the common cold, even if some of the symptoms are similar to those of flu.
2. What you need to know before you start using Efluelda
To ensure that Efluelda is suitable for you, it is important that you inform your doctor or pharmacist if any of the following points apply to you. If there is anything you do not understand, ask your doctor or pharmacist to explain it to you.
Do not use Efluelda:
- if you are allergic to:
- the active substances, or
- any of the other components of this vaccine (listed in section 6), or
- any of the components that may be present in minimal amounts, such as eggs (ovoalbumin, chicken proteins) and formaldehyde.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Efluelda.
Consult your doctor before being vaccinated if you:
- have a weakened immune system (immunodeficiency or are taking medicines that affect the immune system),
- have bleeding problems or bruise easily,
- have experienced Guillain-Barré Syndrome (severe muscle weakness) after receiving a flu vaccine,
- have a high or moderate fever or an acute illness, vaccination should be postponed until you have recovered.
Your doctor will decide if you should receive the vaccine.
A faint may occur after, or even before, any injection with a needle. Therefore, inform your doctor or nurse if you have fainted with a previous injection.
Like all vaccines, Efluelda may not fully protect all people who are vaccinated.
If you have a blood test a few days after flu vaccination, please inform your doctor. This is because false-positive results have been observed in serological tests in some recently vaccinated patients.
Children
This vaccine must not be used in children, it is only for adults aged 60 years or older.
Other medicines and Efluelda
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines or vaccines.
- If Efluelda is to be given at the same time as other vaccines, the vaccines should always be given in different limbs.
- It should be noted that adverse reactions can be intensified by any co-administration.
- The immune response may be reduced in the case of immunosuppressive treatments, such as corticosteroids, cytotoxic drugs, or radiotherapy.
Pregnancy and breastfeeding
Efluelda is only indicated for use in adults aged 60 years and older.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this vaccine. Your doctor/pharmacist will help you decide if you should receive Efluelda.
Driving and using machines
Efluelda has a negligible influence on the ability to drive and use machines. However, if you feel unwell or dizzy, it is not recommended to drive.
Efluelda contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
3. How to use Efluelda
Adults aged 60 years and older receive a dose of 0.5 ml.
How Efluelda is administered
Your doctor, pharmacist, or nurse will administer the recommended dose of the vaccine as an injection into the muscle or under the skin.
If you have any questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Allergic reactions
Consult your doctor IMMEDIATELYif you experience:
- Severe allergic reactions:
- that can lead to a medical emergency with low blood pressure, shortness of breath, wheezing, or difficulty breathing, rapid heartbeat, and weak pulse, cold, clammy skin (cold sweat), dizziness, which can lead to collapse (anaphylaxis [including angioedema, e.g. swelling more apparent on the face and neck, including face, lips, tongue, throat, or any other part of the body and which can cause difficulty swallowing or breathing]).
Consult a doctor if you experience:
- Allergic reactions such as skin reactions that can spread throughout the body including itching, hives, rash.
These side effects are rare (may affect up to 1 in 1,000 people).
Other reported adverse effects
The following adverse effects were reported in adults aged 60 years and older.
Very common (may affect more than 1 in 10 people):
- Reactions at the injection site: pain, redness (erythema)
- General feeling of being unwell (malaise), headache, muscle pain (myalgia)
Common (may affect up to 1 in 10 people):
- Reactions at the injection site: swelling, bruising, hardness (induration)
- Fever, chills (shivering)
Uncommon (may affect up to 1 in 100 people):
- Reactions at the injection site: itching
- Fatigue, lethargy, feeling sick (nausea), vomiting, diarrhea
- Cough, muscle weakness, indigestion (dyspepsia), sore throat (oropharyngeal pain)
Rare (may affect up to 1 in 1,000 people):
- Lack of energy (asthenia), flushing, joint pain (arthralgia), dizziness, night sweats, rash, numbness or tingling (paresthesia), runny nose (rhinorrhea), vertigo, excessive blood in the white of the eye (ocular hyperemia)
- Pain in the limbs
Frequency not known: frequency cannot be estimated from the available data
- Reduction in the number of certain types of particles in the blood called platelets; a low number of these can result in excessive bruising or bleeding (thrombocytopenia)
- Swelling of the glands in the neck, armpit, or groin (lymphadenopathy)
- Nervous system disorders that can result in stiffness of the neck, confusion, numbness, pain, and weakness of the limbs, loss of balance, loss of reflexes, paralysis of part or all of the body (encephalomyelitis and transverse myelitis, brachial neuritis, Guillain-Barré Syndrome), facial paralysis (Bell's palsy), vision disorders due to optic nerve dysfunction (optic neuritis/optic neuropathy), seizures (including febrile seizures), fainting (syncope) shortly after vaccination
- Inflammation of blood vessels (vasculitis) that can cause skin rashes and, in very rare cases, temporary kidney problems, dilation of blood vessels (vasodilation)
- Chest pain
- Wheezing, feeling of tightness in the throat, difficulty breathing (dyspnea)
Most side effects usually occurred within 3 days after vaccination and resolved within 3 days. The intensity of these side effects was mild to moderate.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Efluelda
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the label and on the carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze. Keep the syringe in the outer packaging to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Efluelda
- The active substances are: Influenza virus (inactivated, split) of the following strains *:
Strain similar to A/Victoria/4897/2022 (H1N1)pdm09: (IVR-238)....... 60 micrograms HA**
Strain similar to A/Croatia/10136RV/2023 (H3N2): (X-425A)…….…60 micrograms HA **
Strain similar to B/Austria/1359417/2021: B/Michigan/01/2021....……60 micrograms HA**
Per 0.5 ml dose
- grown in embryonated chicken eggs
** hemagglutinin
This vaccine complies with the recommendations of the WHO (World Health Organization) (Northern Hemisphere) and the EU decision for the 2025/2026 campaign.
The other components are: a buffer solution containing sodium chloride, sodium phosphate monobasic, sodium phosphate dibasic, water for injections, and octoxinol-9.
Some components such as eggs (ovoalbumin, chicken proteins) or formaldehyde may be present in very small amounts (see Section 2).
Appearance of Efluelda and pack contents
The vaccine, after being carefully shaken, is a colorless, opalescent liquid.
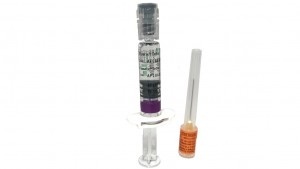
Efluelda is an injectable suspension of 0.5 ml presented in a pre-filled syringe (Injectable suspension) with or without a needle (in packs of 1, 5, or 10) or with a safety needle (in packs of 1 or 10). Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
The marketing authorization holder is:
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly
France
The manufacturer is:
Sanofi Wintrop Industrie
Voie de l’Institut - Parc Industriel d’Incarville
B.P 101
27100 Val de Reuil
France
Local representative
sanofi-aventis, S.A.
C/ Rosselló i Porcel, 21
08016 Barcelona
Spain
Tel: +34 93 485 94 00
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria, Belgium, Bulgaria, Czech Republic, Germany, Denmark, Estonia, Finland, France, Croatia, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, Spain | Efluelda |
Cyprus, Greece | Efluelda TIV |
Date of last revision of this leaflet: July 2025
Other sources of information
The latest approved information on this medicinal product is available by scanning the QR code included in the packaging or at the following internet address: https://efluelda-nh.info.sanofi
-----------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
As with all injectable vaccines, appropriate medical treatment and supervision should be available in case of an anaphylactic episode following administration of the vaccine.
The vaccine should reach room temperature before use.
Shake before use. Inspect visually before administration.
The vaccine should not be used if it contains foreign particles in the suspension.
Do not mix with other medicines in the same syringe.
This vaccine should not be injected directly into any blood vessel.
See also Section 3. How to use Efluelda
Preparation for administration
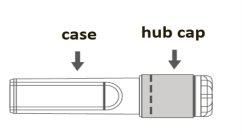
Instructions for use of the safety needle with the Luer Lock syringe:
Image A: Safety needle (inside the bar) | Image B: Components of the safety needle (prepared for use) |
|
|
Step 1:To attach the needle to the syringe, remove the central cap to expose the needle bar, and gently turn the needle in the Luer Lock adapter of the syringe until you feel a slight resistance. |
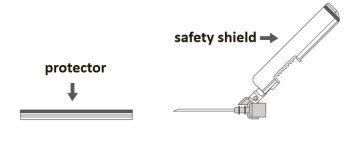
Step 2:Remove the protector from the safety needle. The needle is covered by the safety device and the protector. |
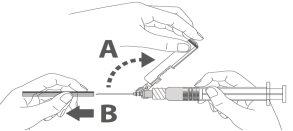
Step 3: A:Separate the safety device from the needle towards the body of the syringe at the angle shown. B:Remove the protector in a straight line. |
|
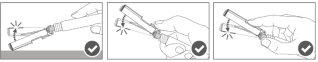
Step 4:Once the injection is complete, lock (activate) the safety device using one of the three techniques illustrated (3) with one hand: activation with a surface, with the thumb, or with the index finger. Note: Activation is verified by a "click" sound and/or tactile. |
|
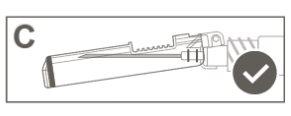
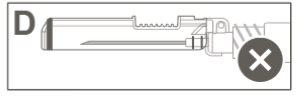
Step 5:Visually inspect the activation of the safety device. The safety device should be completely locked (activated)as shown in figure C. Figure D shows that the safety device is NOTcompletely locked (not activated). |
|
Caution: Do not attempt to unlock (deactivate) the safety device by forcing the needle out of the safety device. |
The disposal of unused vaccines and all materials that have come into contact with them will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EFLUELDA SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 3.75 microgramsActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 60 micrograms of HAActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 15 microgramsActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Seqirus Netherlands B.V.Prescription required
Online doctors for EFLUELDA SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about EFLUELDA SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions