
Efluelda Tetra

Ask a doctor about a prescription for Efluelda Tetra

How to use Efluelda Tetra
PATIENT INFORMATION LEAFLET
Information leaflet attached to the packaging: information for the user
Efluelda Tetra, suspension for injection in a pre-filled syringe
Quadrivalent influenza vaccine (split virion, inactivated),
60 micrograms HA/dose
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. The user of the medicinal product can also help by reporting any adverse reactions that occur after administration of the medicinal product. To find out how to report adverse reactions, see section 4.
Read the leaflet carefully before using the vaccine, as it contains important information for the adult or child patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This vaccine has been prescribed specifically for you. Do not pass it on to others.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist or nurse. See section 4.
Contents of the leaflet
- 1. What Efluelda Tetra is and what it is used for
- 2. Important information before using Efluelda Tetra
- 3. How to use Efluelda Tetra
- 4. Possible side effects
- 5. How to store Efluelda Tetra
- 6. Contents of the pack and other information
1. What Efluelda Tetra is and what it is used for
Efluelda Tetra is a vaccine. This vaccine helps protect people aged 60 and over against flu. The use of Efluelda Tetra should be based on official recommendations for flu vaccination.
After administration of Efluelda Tetra, the immune system (the body's natural defense system) produces its own protection against the disease (antibodies). None of the vaccine components can cause flu.
Flu is an infectious respiratory disease caused by flu viruses, ranging from mild to severe and can lead to serious complications, such as pneumonia, which can result in hospitalization and even death. Flu is a disease that can spread quickly and is caused by many different strains of the virus, which can change every year. Due to this possible annual change in circulating strains as well as the duration of protection provided by the vaccine, it is recommended to get vaccinated every year. The greatest risk of getting flu is during the cold months between October and March. If you have not been vaccinated in the fall, it is still justified to get vaccinated during the spring, as there is still a risk of getting flu. Your doctor will be able to recommend the best time to get vaccinated.
Efluelda Tetra vaccine is intended to protect against the four flu virus strains contained in the vaccine about 2 to 3 weeks after vaccination. Since the incubation period for flu is a few days, if you are exposed to the flu virus shortly before or after vaccination, you can still get the flu.

The vaccine does not protect against the common cold, even though some of its symptoms are similar to flu.
2. Important information before using Efluelda Tetra
Tell your doctor or pharmacist if any of the following apply to the person who is to receive Efluelda Tetra. If anything is unclear, ask your doctor or pharmacist to explain.
When not to use Efluelda Tetra:
- If the person is allergic to:
- active substances, or
- any of the other ingredients of this vaccine (listed in section 6), or
- any ingredient that may be present in very small amounts, such as egg residue (egg albumen, chicken protein) and formaldehyde.
Warnings and precautions
Before using Efluelda Tetra, talk to your doctor, pharmacist or nurse.
Before vaccination, tell your doctor if you have:
- weakened immune response (due to immune deficiency or taking drugs that affect the immune system),
- bleeding or bruising easily,
- previously had Guillain-Barré Syndrome (GBS) (severe muscle weakness) after receiving a flu vaccine,
- an illness with high or moderate fever or acute illness. Vaccination should be postponed until recovery. The doctor will decide whether to administer the vaccine.
Fainting can occur after, or even before, any needle injection. Therefore, tell your doctor or nurse if you have fainted after previous injections.
As with any vaccine, Efluelda Tetra may not provide full protection for all vaccinated individuals.
Tell your doctor if you are planning to have a blood test within a few days after flu vaccination, due to false-positive blood test results observed in some patients who have been vaccinated.
Children
This vaccine should not be used in children. The use of this vaccine is recommended for adults aged 60 and over.
Efluelda Tetra and other medicines
Tell your doctor or pharmacist about all medicines or vaccines you are taking, or have recently taken, and about any medicines or vaccines you plan to take.
- When administering Efluelda Tetra at the same time as other vaccines, the vaccines should always be administered in different limbs.
- Note that side effects may be intensified when vaccines are administered simultaneously.
- When using immunosuppressive drugs, such as corticosteroids, cytotoxic drugs or radiation therapy, the immune response to vaccination may be weakened.
Pregnancy and breastfeeding
Efluelda Tetra is indicated for use only in adults aged 60 and over.
If you are pregnant or breastfeeding, think you may be pregnant or plan to have a child, ask your doctor or pharmacist for advice before using this vaccine. The doctor or pharmacist will help decide whether you should receive Efluelda Tetra.
Driving and using machines
Efluelda Tetra has no or negligible influence on the ability to drive and use machines.
Efluelda Tetra contains potassium and sodium
This vaccine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
3. How to use Efluelda Tetra
Adults aged 60 and over receive one dose of 0.7 ml.
How to administer Efluelda Tetra
Your doctor or nurse will administer the recommended dose of the vaccine as an injection into the muscle or under the skin.
If you have any further questions about the use of this vaccine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Allergic reactions
You should IMMEDIATELYcontact your doctor if you experience:
- Severe allergic reactions:
- which may require medical attention, with low blood pressure, shortness of breath, wheezing, rapid heartbeat and weak pulse, cold, sweaty skin, dizziness which may lead to fainting (anaphylaxis [including angioedema i.e. swelling most noticeable in the face, lips, tongue, throat or other parts of the body, which may cause difficulty in swallowing or breathing]).
Contact your doctor if you experience:
- Allergic reactions such as skin reactions, which can affect the whole body, including itching, hives, rash. These side effects are rare (may affect up to 1 in 1000 people).
Other side effects
The following side effects have been reported in adults aged 60 and over.
Very common (may affect more than 1 in 10 people):
- Injection site reactions: pain, redness (erythema)
- General malaise (fatigue), headache, muscle pain
Common (may affect up to 1 in 10 people):
- Injection site reactions: swelling, bruising, induration
- Fever, chills
Uncommon (may affect up to 1 in 100 people):
- Injection site reactions: itching
- Fatigue, lethargy, nausea, vomiting, diarrhea
- Cough, muscle weakness, indigestion, pharyngitis (sore throat)
Rare (may affect up to 1 in 1000 people):
- Lack of energy (weakness), redness, joint pain, dizziness of central origin, night sweats, rash, numbness or tingling (paresthesia), rhinitis (nasal inflammation), dizziness (balance disorders), excessive blood in the white of the eye (conjunctival congestion)
- Limb pain
Frequency not known: frequency cannot be estimated from the available data
- Decreased number of certain types of blood cells called platelets; their low level can cause excessive bruising or bleeding (thrombocytopenia)
- Enlargement of lymph nodes in the neck, armpit or groin (lymphadenopathy)
- Neurological disorders, which can cause stiffness of the neck, disorientation, numbness, pain and weakness of the limbs, loss of balance, loss of reflexes, paralysis of part or all of the body (encephalitis and transverse myelitis, brachial neuritis, Guillain-Barré syndrome), facial paralysis (Bell's palsy), vision disorders caused by dysfunction of the optic nerves (optic neuritis), seizures (convulsions, including febrile convulsions), fainting soon after vaccination
- Vasculitis, which can lead to skin rashes and in very rare cases to transient kidney function disorders, vasodilation
- Chest pain
- Wheezing, throat constriction, difficulty breathing (dyspnea)
Most side effects occurred usually within 3 days after vaccination and resolved within 3 days. The intensity of these side effects was mild to moderate.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Efluelda Tetra
Store the vaccine out of sight and reach of children.
Do not use this vaccine after the expiry date stated on the label and carton after "Expiry Date (EXP)". The expiry date refers to the last day of the month.
Store in a refrigerator (2°C - 8°C). Do not freeze. Store the pre-filled syringe in the outer packaging to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Efluelda Tetra contains
- The active substances are: Influenza virus (inactivated, split) of the following strains*:
A/Victoria/4897/2022 (H1N1)pdm09-like strain (A/Victoria/4897/2022, IVR-238)
.............................................................................................................................. 60 micrograms HA**
A/Croatia/10136RV/2023 (H3N2)-like strain (A/Croatia/10136RV/2023, X-425A)
.............................................................................................................................. 60 micrograms HA**
B/Austria/1359417/2021-like strain (B/Michigan/01/2021, wild type)
……………………………………….. ................................................................ 60 micrograms HA**
B/Phuket/3073/2013-like strain (B/Phuket/3073/2013, wild type)
….…………………………………….. .............................................................. 60 micrograms HA**
in a 0.7 ml dose
*
propagated in chicken embryos
**
haemagglutinin
The vaccine is in accordance with the recommendations of the World Health Organization (WHO) for the Northern Hemisphere and with the recommendations of the European Union for the 2025/2026 season.
The other ingredients are: buffer solution containing sodium chloride, disodium phosphate, sodium dihydrogen phosphate, water for injection and octoxynol-9.
Some ingredients such as egg residues (egg albumen, chicken protein) or formaldehyde may be present in very small amounts (see section 2).
What Efluelda Tetra looks like and contents of the pack
After gentle agitation, the vaccine is a colorless, opalescent liquid.
Efluelda Tetra is a suspension for injection in a pre-filled syringe of 0.7 ml, with or without a needle (in packs of 1, 5 or 10) or with a needle in a protective shield (in packs of 1 or 10). Not all pack sizes may be marketed.
Marketing authorization holder and importer
Marketing authorization holder:
Sanofi Winthrop Industrie
82 Avenue Raspail
94250 Gentilly, France
Importer:
Sanofi Winthrop Industrie
Voie de l’Institut - Parc Industriel d'Incarville
B.P 101
27100 Val de Reuil, France
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Date of last revision of the leaflet:July 2025
Other sources of information
Currently approved information about this product is available by scanning the QR code on the carton or at the following URL:
https://eflueldatetra-nh.info.sanofi/
Information intended for healthcare professionals only:
As with all injectable vaccines, appropriate medical treatment and supervision should be available in case of anaphylactic reaction following administration of the vaccine.
Before administration, the vaccine should be at room temperature.
Shake before use. Check visually before administration.
The vaccine should not be used if particles are present in the suspension.
The vaccine should not be mixed with other medicinal products in the same syringe.
The vaccine should not be injected directly into blood vessels.
See also section 3. How to use Efluelda Tetra
| Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Germany, Denmark, Greece, Finland, France, Croatia, Hungary, Ireland, Italy, Latvia, Netherlands, Norway, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, Spain | Efluelda Tetra |
| United Kingdom (Northern Ireland) | Quadrivalent Influenza Vaccine (Split Virion, Inactivated) High Dose |
Preparation for administration
Instructions for using the safety needle shield with the pre-filled syringe with Luer Lock tip:
| Figure A: Safety needle shield (in housing) | Figure B: Safety needle shield components (ready for use) |
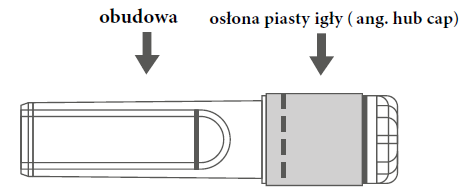 |  |
| Step 1: To attach the needle to the syringe, remove the hub cap to expose the hub and gently screw the needle into the Luer Lock adapter of the syringe until a slight resistance is felt. | |
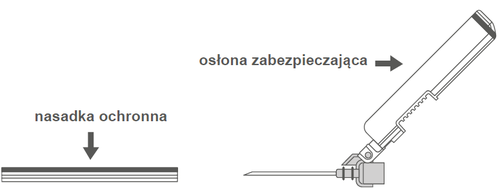
Step 2: Remove the needle shield. The needle is protected by a safety shield and a protective cap.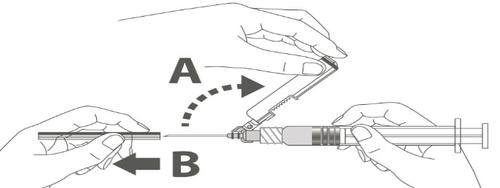 | |
| Step 3: A: Slide the safety shield away from the needle in the direction of the syringe body at the shown angle. B: Remove the protective cap. | |
| Step 4: After injection, lock (activate) the safety shield by using one of the three (3) one-handed techniques: activation on a flat surface, with the thumb or index finger. Note: Activation is confirmed by an audible and/or palpable "click". | 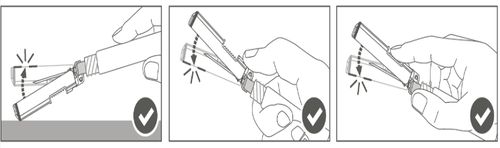 |
| Step 5: Visually check the operation of the safety shield. The safety shield should be fully locked (activated), as shown in Figure C. Figure D shows that the safety shield is NOT fully locked (not activated). | 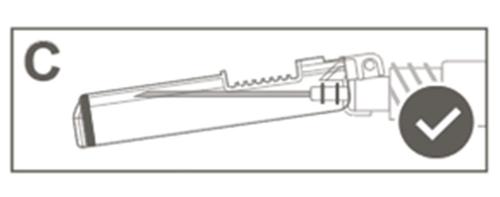 |
Warning: Do not attempt to unlock (deactivate) the safety device by pushing the needle out of the safety shield.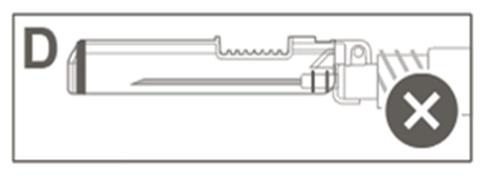 | |
Any unused product or waste material should be disposed of in accordance with local requirements.>
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterSanofi Winthrop Industrie
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Efluelda TetraDosage form: Suspension, 60 mcg HA/strain, 1 dose (0.5 ml)Active substance: influenza, inactivated, split virus or surface antigenPrescription requiredDosage form: Suspension, 1 dose (0.5 ml)Active substance: influenza, inactivated, split virus or surface antigenPrescription requiredDosage form: Suspension, 1 dose (0.5 ml)Active substance: influenza, inactivated, split virus or surface antigenPrescription required
Alternatives to Efluelda Tetra in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Efluelda Tetra in Spain
Alternative to Efluelda Tetra in Ukraine
Online doctors for Efluelda Tetra
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Efluelda Tetra – subject to medical assessment and local rules.














