
FLIXOTIDE ACCUHALER 500 micrograms/inhalation, powder for inhalation

How to use FLIXOTIDE ACCUHALER 500 micrograms/inhalation, powder for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- fluticasone propionate
- What Flixotide Accuhaler 500 micrograms/inhalation is and what it is used for
- What you need to know before you use Flixotide Accuhaler 500 micrograms/inhalation
- How to use Flixotide Accuhaler 500 micrograms/inhalation
- Possible side effects
- Storing Flixotide Accuhaler 500 micrograms/inhalation
- Packaging Content and Additional Information
Introduction
Package Leaflet: Information for the User
Flixotide Accuhaler 500 micrograms/inhalation, powder for inhalation
fluticasone propionate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Flixotide Accuhaler 500 micrograms/inhalation is and what it is used for
- What you need to know before you use Flixotide Accuhaler 500 micrograms/inhalation
- How to use Flixotide Accuhaler 500 micrograms/inhalation
- Possible side effects
- Storing Flixotide Accuhaler 500 micrograms/inhalation
- Contents of the pack and other information
1. What Flixotide Accuhaler 500 micrograms/inhalation is and what it is used for
Fluticasone propionate belongs to a group of medicines called corticosteroids. Corticosteroids are used to treat asthma because of their anti-inflammatory action. They reduce inflammation and irritation in the walls of the small airways in the lungs and so help to control asthma symptoms. Corticosteroids also help to prevent asthma attacks.
Flixotide Accuhaler 500 micrograms/inhalation is recommended for the treatment of adults with moderate to severe asthma and chronic obstructive pulmonary disease (COPD).
2. What you need to know before you use Flixotide Accuhaler 500 micrograms/inhalation
Do not useFlixotide Accuhaler 500 micrograms/inhalation
- if you are allergic (hypersensitive) to fluticasone propionate or any of the other ingredients of this medicine listed in section 6.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you start using Flixotide Accuhaler 500 micrograms/inhalation:
- if you have ever had to stop using this or any other medicine for your condition because of side effects
- if you notice that you are becoming increasingly wheezy or short of breath
- if you have ever had pulmonary tuberculosis
- if you are taking or have recently taken other corticosteroid medicines by mouth or by inhalation
- if you have an infection
- if you are under stress, such as due to a severe illness, surgical procedure, or injury, your dose of Flixotide may need to be increased
- if you have diabetes mellitus (Flixotide may increase blood sugar levels)
- if your breathing worsens after using this medicine, stop using it immediately and tell your doctor as soon as possible
Flixotide should not be used to relieve acute asthma attacks, but only as a long-term treatment to control symptoms. The treatment should not be stopped abruptly.
Contact your doctor if you experience blurred vision or other visual disturbances.
Children and adolescents
Flixotide Accuhaler 500 micrograms/inhalation is not indicated for use in children. Other forms of Flixotide are more suitable for children and adolescents under 16 years of age.
Using Flixotide Accuhaler 500 micrograms/inhalation with other medicines
Tell your doctor or pharmacist if you are using or have recently used any other medicines, including those obtained without a prescription.
Some medicines may increase the effects of Flixotide Accuhaler 500 micrograms/inhalation, and your doctor may wish to monitor you closely if you are using these medicines (including some medicines for HIV: ritonavir, cobicistat).
Oral medicines for the treatment of fungal infections (ketoconazole).
No interactions with other medicines have been reported. However, you should tell your doctor or pharmacist if you are taking or have taken medicines for the treatment of tuberculosis.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Flixotide is unlikely to affect your ability to drive or use machines.
Flixotide Accuhaler 500 micrograms/inhalation contains lactose. This may cause an allergic reaction in patients with a cow's milk protein allergy. If your doctor has told you that you have an intolerance to some sugars, contact them before using this medicine.
Warning for athletes
Athletes should note that this medicine contains an active substance that may cause a positive result in doping tests.
3. How to use Flixotide Accuhaler 500 micrograms/inhalation
Use Flixotide Accuhaler 500 micrograms/inhalation exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
Remember to use your medicine.
Your doctor will tell you how long to use Flixotide Accuhaler 500 micrograms/inhalation. Do not stop using Flixotide Accuhaler 500 micrograms/inhalation without talking to your doctor first.
It is very important to inhale each dose exactly as your doctor has told you. The usual dose is:
- ASTHMA
Adults and adolescents over 16 years of age: 1-2 inhalations twice daily (500-1000 micrograms twice daily).
- COPD
Adults: 1 inhalation twice daily (500 micrograms twice daily).
The contents of the inhalations should not be swallowed, but inhaled to reach the lungs. You may not be able to taste or feel the powder in your mouth, even if you have used the Accuhaler correctly. If you have any doubts, ask your doctor or pharmacist.
Do not inhale more doses or use Flixotide Accuhaler 500 micrograms/inhalation more often than your doctor has told you.
This medicine may take some time to work. It is very important to use it regularly. If your breathing or wheezing gets worse after using Flixotide Accuhaler 500 micrograms/inhalation, stop using it and tell your doctor immediately. If your symptoms do not improve or get worse after 7 days, tell your doctor.
Do not use this medicine to treat a sudden attack of wheezing or shortness of breath. You will need a different medicine to treat such attacks. If you are using more than one medicine, be careful not to confuse them.
If you think that Flixotide Accuhaler 500 micrograms/inhalation is too strong or too weak, tell your doctor or pharmacist.
Instructions for the correct use of Flixotide Accuhaler 500 micrograms/inhalation
The device is wrapped in a foil wrapper to keep it clean and dry. This should only be opened when you are ready to use it for the first time. Once opened, the wrapper should be discarded. The device has two positions: closed and open.
CLOSED
When you open the case and take the device out of the foil wrapper for the first time, it will be in the closed position.
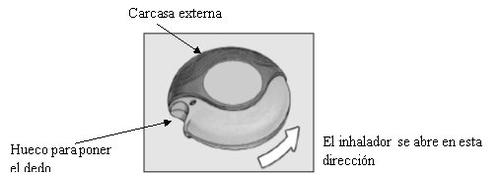
OPEN
The device contains 60 individual doses of the medicine in powder form. The dose counter shows how many doses are left.
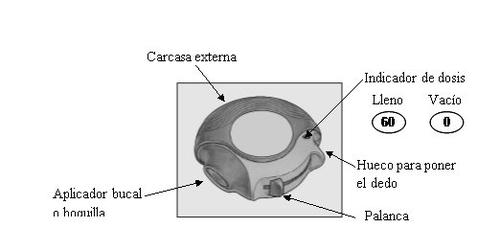
Each dose is precisely measured and hygienically protected. No maintenance or refilling is required.
The dose counter, located on the top of the device, shows how many doses are left. The numbers from 5 to 0 will appear in red to warn you that there are only a few doses left.
Using the device is easy. When you need a dose, follow these five simple steps:
- Open.
- Slide.
- Inhale.
- Close.
- Rinse.
Device operation
When you slide the lever on the device, a small hole in the mouthpiece opens and a dose is prepared for inhalation. When you close the device, the lever automatically returns to its original position, ready for the next dose. The outer casing protects the device when not in use.
- Open:
To open the device, hold the outer casing with one hand and place your thumb in the thumb grip with your other hand. Push your thumb away from you as far as it will go.
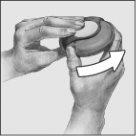
- Slide:
Hold the device with the mouthpiece towards you. Slide the lever away from you as far as it will go – you will hear a click. The device is now ready for use. Each time the lever is pulled back, a dose is prepared for inhalation, as shown by the dose counter. Do not play with the lever, as this will prepare doses that will be wasted.
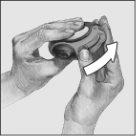
- Inhale:
- Before you start to inhale a dose, read this section carefully.
- Hold the device away from your mouth. Breathe out gently – do not breathe out into the device.
- Put the mouthpiece in your mouth. Take a steady, deep breath in through the device – do not breathe in through your nose.
- Take the device out of your mouth and hold your breath for 10 seconds or as long as you comfortably can.
- Breathe out slowly.

- Close:
To close the device, put your thumb in the thumb grip and slide it back towards you as far as it will go.
When you close the device, you will hear a click. The lever will automatically return to its original position, ready for the next dose.
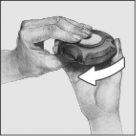
- Rinse:
After use, rinse your mouth with water and spit it out.
If your doctor has prescribed two inhalations, close the device and repeat steps A to E.
Remember
Keep the device dry.
Keep the device closed when not in use.
Do not breathe out into the device.
Slide the lever only when you are ready to inhale a dose.
Ask your doctor or pharmacist if you have any doubts.
If you use more Flixotide Accuhaler 500 micrograms/inhalation than you should
If you have used more Flixotide Accuhaler 500 micrograms/inhalation than you should, contact the Poisons Information Service (telephone 91 562 04 20) or talk to your doctor or pharmacist immediately. Take this leaflet with you.
If you forget to use Flixotide Accuhaler 500 micrograms/inhalation
Do not inhale a double dose to make up for a forgotten dose. If you forget a dose, just take the next dose at the usual time.
If you stop using Flixotide Accuhaler 500 micrograms/inhalation
It is very important that you use Flixotide Accuhaler 500 micrograms/inhalation every day as prescribed by your doctor. Do not stop using Flixotide Accuhaler 500 micrograms/inhalation without talking to your doctor first, as your symptoms may get worse. Do not stop using Flixotide Accuhaler 500 micrograms/inhalation suddenly.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some people may develop a fungal infection in the mouth and throat (candidiasis) and experience soreness in the throat or hoarseness after inhaling this medicine. To avoid this, it is recommended to brush your teeth or rinse your mouth or gargle with water after each dose. If you notice any of these symptoms, tell your doctor but do not stop using Flixotide Accuhaler 500 micrograms/inhalation unless your doctor tells you to.
If you experience any of the following symptoms after using Flixotide Accuhaler 500 micrograms/inhalation, stop using it and tell your doctor immediately:
- sudden onset of wheezing or chest tightness
- swelling of the eyelids, face, lips, tongue, or throat
- skin rash or redness (hives) or itching in any part of the body
Other side effects include:
Very common (may affect more than 1 in 10 people)
- candidiasis (fungal infection) in the mouth and throat
Common (may affect up to 1 in 10 people)
- pneumonia (infection of the lungs) and bronchitis (inflammation of the airways) in patients with chronic obstructive pulmonary disease (COPD)
Tell your doctorif you have any of the following symptoms while using Flixotide Accuhaler 500 micrograms/inhalation. They could be symptoms of a lung infection:
- fever or chills
- increased production of mucus, change in the color of mucus
- increased coughing or difficulty breathing
- hoarseness
- bruising
Uncommon (may affect up to 1 in 100 people)
- skin hypersensitivity reactions
Rare (may affect up to 1 in 1,000 people)
- oesophageal candidiasis
Very rare (may affect up to 1 in 10,000 people)
- angioedema (skin reactions with redness, swelling, and itching), mainly facial and oropharyngeal oedema
- respiratory symptoms such as dyspnoea (shortness of breath) and/or bronchospasm (narrowing of the airways)
- anaphylactic reactions (severe allergic reactions that can cause a sudden drop in blood pressure and loss of consciousness)
- moon face (Cushing's syndrome)
- suppression of the activity of the adrenal gland, which can cause tiredness, weight loss, nausea, vomiting, headache, low blood pressure, numbness, and convulsions
- growth retardation in children and adolescents
- decreased bone density
- cataracts and glaucoma
- hyperglycaemia (high blood sugar levels). If you have diabetes, you may need to check your blood sugar levels more often and adjust your usual treatment for controlling your diabetes
- anxiety, sleep disturbances, and behavioral changes, including hyperactivity and irritability (mainly in children)
- paradoxical bronchospasm (temporary narrowing of the airways after using the inhaler)
Frequency not known
- nasal bleeding
- blurred vision
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Agency: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Flixotide Accuhaler 500 micrograms/inhalation
Do not store above 30°C. Store in the original packaging to protect from moisture.
Keep the device in the foil wrapper until you are ready to use it for the first time. Discard the wrapper once opened.
Keep out of the sight and reach of children.
Do not use Flixotide Accuhaler 500 micrograms/inhalation after the expiry date stated on the packaging after EXP. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Packaging Content and Additional Information
Composition ofFlixotide Accuhaler 500 micrograms/inhalation
- The active ingredient is 500 micrograms of fluticasone propionate per inhalation (alveolus).
- The other component is lactose monohydrate (contains milk proteins).
Appearance of the Product and Packaging Content
Packaging with a device containing 60 alveoli with powder for oral inhalation. The device is packaged inside an aluminum wrapper.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Manufacturer:
Glaxo Wellcome Production
Zone Industrielle Nº2
23 Rue Lavoisier,
27000 Evreux
France
Date of the Last Revision of this Leaflet:January 2022.
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price31.47 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLIXOTIDE ACCUHALER 500 micrograms/inhalation, powder for inhalationDosage form: PULMONARY INHALATION, 250 mcg fluticasone propionateActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 mcg fluticasone propionateActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 100 µgActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for FLIXOTIDE ACCUHALER 500 micrograms/inhalation, powder for inhalation
Discuss questions about FLIXOTIDE ACCUHALER 500 micrograms/inhalation, powder for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















