
FARYDAK 20 mg HARD CAPSULES

How to use FARYDAK 20 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Farydak 10 mg Hard Capsules
Farydak 15 mg Hard Capsules
Farydak 20 mg Hard Capsules
panobinostat
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because
it contains important information for you
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Farydak and what is it used for
- What you need to know before you take Farydak
- How to take Farydak
- Possible side effects
- Storage of Farydak
- Contents of the pack and other information
1. What is Farydak and what is it used for
What is Farydak
Farydak is an anticancer medicine that contains the active substance panobinostat, which belongs to a group of medicines called pan-deacetylase inhibitors.
What Farydak is used for
Farydak is used to treat adult patients who have a rare type of blood cancer called multiple myeloma. Multiple myeloma is a disorder of plasma cells (a type of blood cell) that grow out of control in the bone marrow.
Farydak blocks the growth of cancerous plasma cells and reduces their number.
Farydak is always used with two other medicines: bortezomib and dexamethasone.
If you have any questions about how Farydak works or why you have been prescribed it, ask your doctor or pharmacist.
2. What you need to know before you take Farydak
Do not take Farydak:
- if you are allergic to panobinostat or any of the other ingredients of this medicine (listed in section 6).
(including those listed in section 6).
- if you are breast-feeding.
Warnings and precautions
Follow your doctor's instructions carefully
Tell your doctor or pharmacist before you start taking Farydak:
- if you have liver problems or have ever had liver disease.
- if you have heart problems or heart rhythm problems, such as irregular heartbeats or a condition called long QT syndrome.
- if you have a bacterial, viral, or fungal infection.
- if you have gastrointestinal problems such as diarrhea, nausea, or vomiting.
- if you have a blood clotting disorder.
Tell your doctor or pharmacist immediately if you experience any of the following while taking Farydak:
- any signs of gastrointestinal problems.
- any signs of liver problems.
- any signs of infection.
- any signs of heart problems.
The list of associated symptoms is described in section 4, possible side effects.
Your doctor may change your dose of Farydak, temporarily stop treatment, or stop treatment altogether if you experience side effects.
Monitoring during treatment with Farydak
During treatment with Farydak, you will have regular blood tests to:
- check how your liver is working (by measuring blood levels of bilirubin and transaminases, which are substances made in the liver).
- check the levels of certain blood cells (white blood cells, red blood cells, platelets).
- check the levels of electrolytes (such as potassium, magnesium, phosphate) in your body.
- check how your thyroid and pituitary gland are working (by measuring blood levels of thyroid hormones).
Your heart rhythm will also be checked using a device that measures the electrical activity of your heart (called an ECG).
Children and adolescents
Farydak must not be used in children and adolescents under 18 years.
Other medicines and Farydak
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription, such as vitamins, herbal medicines, as they may interact with Farydak.
In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
- medicines used to treat infections, including fungal infections (such as ketoconazole, itraconazole, voriconazole, or posaconazole) and some bacterial infections (antibiotics such as clarithromycin or telithromycin). Medicines used to treat tuberculosis, such as rifabutin or rifampicin.
- medicines used to prevent seizures or fits (antiepileptics such as carbamazepine, phenobarbital, or phenytoin).
- medicines used to treat HIV, such as ritonavir or saquinavir.
- medicines used to treat depression, such as nefazodone.
- St. John's Wort, a herbal medicine used to treat depression.
- medicines used to prevent blood clotting, such as warfarin or heparin.
- medicines used to treat cough, such as dextromethorphan.
- medicines used to treat irregular heart rhythm, such as amiodarone, disopyramide, procainamide, quinidine, propafenone, or sotalol.
- medicines that may have an unwanted effect on the heart (called QT prolongation), such as chloroquine, halofantrine, methadone, moxifloxacin, bepridil, or pimozide.
- medicines used to treat high blood pressure, such as metoprolol or nebivolol.
- medicines used to treat serious mental health problems, such as risperidone.
- medicines used to treat breast cancer, such as tamoxifen.
- medicines used to treat nausea and vomiting, such as dolasetron, granisetron, ondansetron, or tropisetron, as they may also cause unwanted effects on the heart (QT prolongation).
- atomoxetine, a medicine used to treat attention deficit hyperactivity disorder.
These medicines should be used with caution or avoided while taking Farydak. If you are taking any of these medicines, your doctor may prescribe you another medicine during treatment with Farydak.
Ask your doctor or pharmacist if you are not sure if the medicines you are taking are included in the above list.
While taking Farydak, you should also tell your doctor or pharmacist if you are prescribed another medicine that you have not taken before.
Taking Farydak with food and drink
Do not eat starfruit, pomegranate, or grapefruit or drink their juiceswhile taking Farydak, as they may increase the amount of medicine that gets into your blood.
Pregnancy and breast-feeding
Due to the potential risk of death or birth defects, Farydak must not be taken during:
- Pregnancy
Farydak must not be taken during pregnancy unless the potential benefit to the mother outweighs the potential risk to the baby. If you are pregnant, think you may be pregnant, or plan to become pregnant, ask your doctor. Your doctor will inform you of the potential risks of taking Farydak during pregnancy.
- Breast-feeding
Do not take Farydak while breast-feeding.
Contraception in men and women
Due to the potential risk of death or birth defects, you must use the following contraceptive methods while taking Farydak:
- For women taking Farydak
If you are sexually active, you must have a pregnancy test before starting treatment with Farydak and use a highly effective contraceptive method while taking Farydak. You must also continue to use contraception for 3 months after stopping treatment with Farydak. Ask your doctor which method is most suitable for you. If you use a hormonal contraceptive, you must also use a barrier method (such as a condom or diaphragm).
- For men taking Farydak
If you are sexually active, you must use condoms while taking Farydak and for 6 months after stopping treatment. If your partner could become pregnant, she must also use a highly effective contraceptive method during your treatment and for 6 months after stopping treatment. Tell your doctor immediately if your partner becomes pregnant while you are taking Farydak or within 6 months after stopping treatment.
Driving and using machines
Farydak may slightly affect your ability to drive or use machines. If you feel dizzy while taking this medicine, do not drive a vehicle or use tools or machinery.
3. How to take Farydak
Follow your doctor's instructions exactly. If you are unsure, ask your doctor or pharmacist.
How much to take
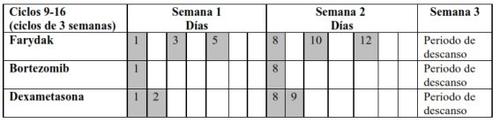
- Farydak is taken for 21 days (2 weeks of treatment followed by 1 week without treatment) – this is called a treatment cycle.
- Do not take the medicine every day.
- Depending on your doctor's recommendations, the dose of Farydak is 20 mg, 15 mg, or 10 mg, taken once daily, on days 1, 3, 5, 8, 10, and 12, of the 21-day cycle.
- Do not take Farydak during week 3.
- After week 3, a new cycle starts again, as explained below in Table 1 and 2.
- Refer to Table 1 for cycles 1-8 and Table 2 for cycles 9-16.
Table 1 Recommended regimen for taking Farydak in combination with bortezomib and dexamethasone (cycles 1-8)
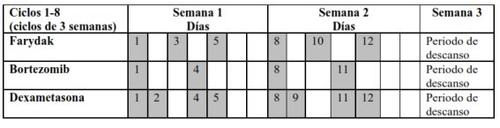
Table 2 Recommended regimen for taking Farydak in combination with bortezomib and dexamethasone (cycles 9-16)
Your doctor will tell you exactly how many Farydak capsules to take. Do not change the dose without talking to your doctor.
Take Farydak once a day, at the same time each day, only on the specified days of each treatment cycle.
How to take this medicine
- Swallow the capsules whole with a glass of water.
- The medicine can be taken with or without food.
- Do not chew or crush the capsules.
If you vomit after taking Farydak, do not take another capsule until your next scheduled dose.
How to use the Farydak blister pack

How long to take Farydak
You should take Farydak for as long as your doctor tells you. It is a long-term treatment with 16 cycles (48 weeks). Your doctor will monitor your condition to see if the treatment is working. If you have questions about how long to take Farydak, ask your doctor or pharmacist.
If you take more Farydak than you should
If you accidentally take more capsules than you should, or if someone else takes your medicine, tell your doctor or go to the nearest hospital immediately. Take the pack and this leaflet with you. You may need medical treatment.
If you forget to take Farydak
- If it is less than 12 hours since you should have taken the medicine, take the missed dose as soon as you remember. Then continue with your normal schedule.
- If it is more than 12 hours since you should have taken the medicine, do not take the missed dose. Continue with your normal schedule.
Do not take a double dose to make up for a missed dose.
Never take a missed dose of Farydak if you are in the part of the cycle where you are not supposed to take any Farydak.
Tell your doctor about all missed doses throughout the 21-day treatment cycle.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
- Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Some adverse effects can be serious
STOP taking Farydak and seek immediate medical help if you notice any of the following:
- difficulty breathing or swallowing, swelling of the face, lips, tongue, or throat, extensive skin irritation, with blisters and rashes (signs of an allergic reaction)
- severe headache, feeling of weakness or paralysis of the limbs or face, difficulty speaking, sudden loss of consciousness (potential signs of nervous system problems such as bleeding or swelling of the skull or brain)
- rapid breathing, feeling of dizziness
- oppressive and sudden chest pain, feeling of fatigue, irregular heartbeat (potential signs of a heart attack)
- coughing up blood, loss of bloody fluid through the nose (signs of bleeding in the lungs)
- vomiting blood, black or bloody stools, passage of fresh blood through the anus, normally in or with stools (signs of gastrointestinal bleeding)
- difficulty breathing with bluish skin around the mouth, which could lead to loss of consciousness (sign of severe lung problems)
- fever, chest pain, increased heart rate, decreased blood pressure, difficulty breathing or agitated breathing (sign of blood poisoning known as sepsis)
- chest pain or discomfort, alterations in heart rhythm (slower or faster), palpitations, feeling of dizziness, fainting, dizziness, bluish discoloration of the lips, difficulty breathing, swelling of the lower limbs or skin (signs of heart problems)
Tell your doctor or pharmacist immediately if you notice any of the following adverse effects:
- abdominal or stomach pain, nausea, diarrhea, vomiting, black or bloody stools, constipation, burning, swelling, or abdominal distension (signs of gastrointestinal problems)
- new symptoms or worsening of symptoms such as cough with or without mucus, fever, difficult or painful breathing, wheezing (breathing sounds), chest pain when breathing, difficulty breathing, feeling of shortness of breath, pain or burning sensation when urinating, excessive urge to urinate, blood in the urine (signs of infection in the lungs or urinary tract)
- fever, sore throat, mouth ulcers due to infections (signs of a low white blood cell count)
- sudden bleeding or bruising under the skin (signs of a low platelet count)
- diarrhea, abdominal pain, fever (signs of inflamed colon)
- feeling of dizziness, especially when standing up (sign of low blood pressure)
- feeling of thirst, low urine output, weight loss, dry, reddened skin, irritability (signs of dehydration)
- swollen ankles (sign of low albumin levels in the blood, also known as hypoalbuminemia)
- feeling of fatigue, itching, yellowish skin and white eye discoloration, nausea or vomiting, loss of appetite, pain in the right upper abdomen, dark or brown urine, bleeding or bruising more frequently than usual (signs of liver problems)
- decreased urine output, swelling in the legs (signs of kidney problems)
- muscle weakness, muscle spasms, irregular pulse (signs of changes in potassium levels in the blood)
Other possible adverse effects
If any of these adverse effects worsen, inform your doctor or pharmacist immediately.
Very common (may affect more than 1 in 10 people)
- feeling of fatigue, pale skin. These signs could indicate a low red blood cell count.
- decreased appetite or weight loss
- difficulty falling asleep or staying asleep (insomnia)
- headache
- feeling of dizziness, fatigue, or weakness
- vomiting, nausea, stomach discomfort, indigestion
- swelling of the legs or arms
- low phosphate or sodium levels in the blood
Common (may affect up to 1 in 10 people)
- small, fluid-filled blisters, skin redness, mouth or gum inflammation (signs of a potentially serious viral infection)
- inflamed ear, nosebleeds or white eye discoloration, bruising, skin inflammation due to infection (rash, skin redness, also known as erythema)
- abdominal pain, diarrhea, swelling, or abdominal distension (signs of stomach mucosa inflammation)
- mouth sores (fungal infection in the mouth)
- feeling of thirst, high urine output, increased appetite with weight loss (signs of high blood sugar levels)
- rapid weight gain, swelling of the hands, ankles, feet, or face (signs of water retention)
- reduced calcium levels in the blood, which can sometimes cause muscle spasms
- uncontrolled body tremors
- palpitations
- abnormal lung sounds when breathing, such as rattling, clicking, or crackling sounds
- chapped and cracked lips
- dry mouth or altered sense of taste
- flatulence
- joint pain or inflammation
- blood in the urine (a sign of kidney problems)
- inability to control urine flow due to weak bladder control
- chills
- weight gain, feeling of fatigue, hair loss, muscle weakness, feeling of cold (signs of an underactive thyroid gland, also known as hypothyroidism)
- general feeling of discomfort
- increased uric acid levels in the blood
- reduced magnesium levels in the blood
- increased creatinine levels in the blood
- high blood levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), or alkaline phosphatase (ALP)
Uncommon (may affect up to 1 in 100 people)
- flat, pin-sized, red or purple skin spots
Reporting Adverse Effects
If you experience any adverse effects, consult your doctor, pharmacist, or nurse, even if they are possible adverse effects not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V.
By reporting adverse effects, you can help provide more information on the safety of this medicine.
5. Storage of Farydak
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiration date shown on the carton and blister.
- Do not store above 30°C.
- Store in the original packaging to protect from moisture.
- Do not use this medicine if you notice that the packaging is damaged or if you notice signs of tampering.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Farydak Composition
- The active ingredient of Farydak is panobinostat.
- Each 10 mg Farydak hard capsule contains 10 mg of panobinostat. The other ingredients are: magnesium stearate, mannitol, microcrystalline cellulose, pregelatinized starch, gelatin, titanium dioxide (E171), brilliant blue FCF (E133), yellow iron oxide (E172), black iron oxide (E172), propylene glycol (E1520), shellac lacquer.
- Each 15 mg Farydak hard capsule contains 15 mg of panobinostat. The other ingredients are: magnesium stearate, mannitol, microcrystalline cellulose, pregelatinized starch, gelatin, titanium dioxide (E171), yellow iron oxide (E172), red iron oxide (E172), black iron oxide (E172), propylene glycol (E1520), shellac lacquer.
- Each 20 mg Farydak hard capsule contains 20 mg of panobinostat. The other ingredients are: magnesium stearate, mannitol, microcrystalline cellulose, pregelatinized starch, gelatin, titanium dioxide (E171), red iron oxide (E172), black iron oxide (E172), propylene glycol (E1520), shellac lacquer.
Farydak Appearance and Package Contents
Farydak 10 mg hard capsules are presented in blisters. They are light green, opaque capsules (15.6-16.2 mm) containing a white or off-white powder. The capsules have a radial mark "LBH 10 mg" in black ink on the cap and two radial marks in black ink on the body.
Farydak 15 mg hard capsules are presented in blisters. They are orange, opaque capsules (19.1-19.7 mm) containing a white or off-white powder. The capsules have a radial mark "LBH 15 mg" in black ink on the cap and two radial marks in black ink on the body.
Farydak 20 mg hard capsules are presented in blisters. They are red, opaque capsules (19.1-19.7 mm) containing a white or off-white powder. The capsules have a radial mark "LBH 20 mg" in black ink on the cap and two radial marks in black ink on the body.
The following package sizes are available: boxes with blisters containing 6, 12, or 24 capsules.
Not all package sizes may be marketed.
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
You can obtain more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Novartis Pharma N.V. Tel: +32 2 246 16 11 Bulgaria Novartis Bulgaria EOOD Tel: +359 2 489 98 28 | Lithuania UAB Novartis Baltics, Lithuanian branch Tel: +370 5 269 16 50 Luxembourg/Luxemburg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 26 37 82 555 |
Estonia Novartis Baltics OÜ, Estonian branch Tel: +372 66 30 810 | Norway Novartis Norge AS Tel: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Poland Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croatia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Tel: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia SIA “Novartis Baltics” Tel: +371 67 887 070 | United Kingdom Novartis Pharmaceuticals UK Ltd. Tel: +44 1276 698370 |
Date of Last Revision of this Leaflet
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FARYDAK 20 mg HARD CAPSULESDosage form: CAPSULE, 10 mgActive substance: panobinostatManufacturer: Pharmaand GmbhPrescription requiredDosage form: CAPSULE, 15 mgActive substance: panobinostatManufacturer: Pharmaand GmbhPrescription requiredDosage form: INJECTABLE PERFUSION, 260 - 500 × 10^6 cellsActive substance: idecabtagene vicleucelManufacturer: Bristol-Myers Squibb Pharma EeigPrescription required
Online doctors for FARYDAK 20 mg HARD CAPSULES
Discuss questions about FARYDAK 20 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






