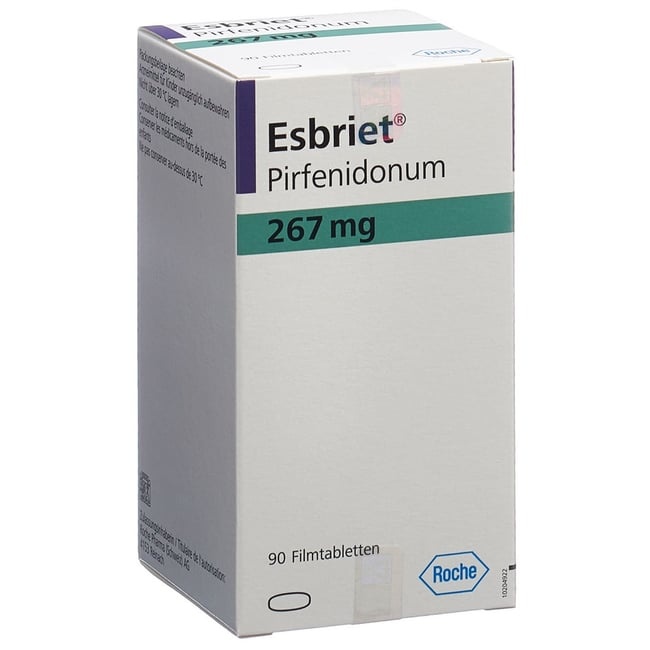
ESBRIET 267 mg FILM-COATED TABLETS

How to use ESBRIET 267 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Esbriet 267mg film-coated tablets
Esbriet 534mg film-coated tablets
Esbriet 801mg film-coated tablets
pirfenidone
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Esbriet and what is it used for
- What you need to know before you take Esbriet
- How to take Esbriet
- Possible side effects
- Storage of Esbriet
- Contents of the pack and other information
1. What is Esbriet and what is it used for
Esbriet contains the active substance pirfenidone and is used for the treatment of Idiopathic Pulmonary Fibrosis (IPF) in adults.
IPF is a disease in which the lung tissue becomes thickened and scarred over time, making it difficult to breathe deeply. In these circumstances, the lungs have difficulty functioning properly. Esbriet helps to reduce scarring and swelling of the lungs, and helps you breathe better.
2. What you need to know before you take Esbriet
Do not take Esbriet
- if you are allergic to pirfenidone or any of the other ingredients of this medicine (listed in section 6)
- if you have previously had angioedema with pirfenidone, including symptoms such as swelling of the face, lips and/or tongue that may be associated with difficulty breathing or wheezing
- if you are taking a medicine called fluvoxamine (used to treat depression and obsessive-compulsive disorder [OCD])
- if you have severe or terminal liver disease
- if you have severe or terminal kidney disease that requires dialysis.
If any of the above applies to you, do not take Esbriet. If you are in doubt, consult your doctor or pharmacist.
Warnings and precautions
Consult your doctor or pharmacist before starting Esbriet
- You may have increased sensitivity to sunlight (photosensitivity reaction) when taking Esbriet. Avoid sun exposure (also UVA lamps) while taking Esbriet. Use a daily sunscreen and cover your arms, legs, and head to reduce sun exposure (see section 4: Possible side effects).
- Do not take other medicines, such as tetracycline antibiotics (e.g., doxycycline), that may increase your sensitivity to sunlight.
- You should inform your doctor if you have kidney problems.
- You should inform your doctor if you have mild to moderate liver problems.
- You should avoid smoking before and during treatment with Esbriet. Smoking may reduce the effect of Esbriet.
- Esbriet may cause dizziness and fatigue. Be careful if you need to perform activities that require attention and coordination.
- Esbriet may cause weight loss. Your doctor will monitor your weight while you are taking this medicine.
- Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported in association with Esbriet treatment. Stop taking Esbriet and seek immediate medical attention if you notice any of the symptoms related to these severe skin reactions described in section 4.
Esbriet may cause serious liver problems. Some cases have been fatal. You will need to have a blood test before starting Esbriet, once a month for the first 6 months, and then every 3 months while taking this medicine, to check your liver function. It is essential that you have these blood tests periodically throughout the time you are taking Esbriet.
Children and adolescents
Do not give Esbriet to children and adolescents under 18 years of age.
Other medicines and Esbriet
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
This is especially important if you are taking the following medicines, as they may alter the effect of Esbriet.
The following medicines may increase the side effects of Esbriet:
- enoxacin (a type of antibiotic)
- ciprofloxacin (a type of antibiotic)
- amiodarone (used to treat certain types of heart conditions)
- propafenone (used to treat certain types of heart conditions)
- fluvoxamine (used to treat depression and obsessive-compulsive disorder [OCD]).
The following medicines may reduce the effectiveness of Esbriet:
- omeprazole (used to treat conditions such as indigestion, gastroesophageal reflux disease)
- rifampicin (a type of antibiotic).
Taking Esbriet with food and drinks
Do not drink grapefruit juice while taking this medicine. Grapefruit juice may make Esbriet not work properly.
Pregnancy and breastfeeding
As a precautionary measure, it is preferable to avoid the use of Esbriet if you are pregnant, planning to become pregnant, or think you may be pregnant, as the potential risks to the fetus are not known.
If you are breastfeeding or planning to breastfeed, talk to your doctor or pharmacist before taking Esbriet. Since it is not known whether Esbriet is excreted in breast milk, your doctor will explain the risks and benefits of taking this medicine during breastfeeding if you decide to do so.
Driving and using machines
Do not drive or operate machinery if you feel dizzy or tired after taking Esbriet.
Esbriet contains sodium
Esbriet contains less than 1 mmol of sodium (23 mg) per tablet, i.e., essentially "sodium-free".
3. How to take Esbriet
Treatment with Esbriet should be initiated and supervised by specialist doctors with experience in the diagnosis and treatment of IPF.
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Normally, you will be given this medicine by gradually increasing the dose as follows:
- for the first 7 days, take a dose of 267 mg (1 yellow tablet), 3 times a day with food (a total of 801 mg/day)
- between days 8 and 14, take a dose of 534 mg (2 yellow tablets or 1 orange tablet), 3 times a day with food (a total of 1,602 mg/day)
- from day 15 (maintenance), take a dose of 801 mg (3 yellow tablets or 1 brown tablet), 3 times a day with food (a total of 2,403 mg/day).
The recommended maintenance dose of Esbriet is 801 mg (3 yellow tablets or 1 brown tablet) three times a day with food, a total of 2,403 mg/day.
Swallow the tablets whole with water, during or after a meal to reduce the risk of side effects such as nausea (feeling sick) and dizziness. If symptoms persist, consult your doctor.
Dose reduction due to side effects
Your doctor may decide to reduce the dose if you experience side effects such as stomach problems, skin reactions to sunlight or UVA lamps, or significant changes in liver enzymes.
If you take more Esbriet than you should
Go to your doctor, pharmacist, or the nearest hospital emergency department immediately if you take more tablets than you should, and take your medicine with you.
If you forget to take Esbriet
If you forget to take a dose, take it as soon as you remember. Do not take a double dose to make up for forgotten doses. Each dose should be separated by an interval of at least 3 hours. Do not take more tablets per day than your prescribed daily dose.
If you stop taking Esbriet
In certain situations, your doctor will advise you to stop taking Esbriet. If you stop taking Esbriet for more than 14 consecutive days for any reason, your doctor will restart your treatment with a dose of 267 mg 3 times a day and gradually increase it to 801 mg 3 times a day.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking Esbriet and seek immediate medical attention if you notice any of the following symptoms or signs:
? Swelling of the face, lips, or tongue, itching, hives, difficulty breathing or wheezing, or feeling faint, which are signs of angioedema, a severe allergic reaction or anaphylaxis.
? Yellowing of the eyes or skin or dark urine, and possibly accompanied by itching of the skin, pain in the upper right side of the abdomen, loss of appetite, bleeding or bruising more easily than normal, or feeling tired. These could be signs of abnormal liver function and may indicate liver damage, which is a rare side effect of Esbriet.
? Red patches, or circular patches on the trunk, often with central blisters, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin rashes may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome or toxic epidermal necrolysis).
? Widespread rash, high body temperature, and enlarged lymph nodes (DRESS or drug reaction with eosinophilia and systemic symptoms).
Other possible side effects are
If you experience any side effect, talk to your doctor.
Very common side effects(may affect more than 1 in 10 people):
- infections of the throat or respiratory tract that reach the lungs and/or sinusitis
- feeling sick (nausea)
- stomach problems, such as acid reflux, vomiting, and constipation
- diarrhea
- indigestion or stomach heaviness
- weight loss
- decreased appetite
- difficulty sleeping
- fatigue
- dizziness
- headache
- difficulty breathing
- cough
- joint pain
Common side effects(may affect up to 1 in 10 people):
- bladder infections
- drowsiness
- altered taste
- hot flashes
- stomach problems, such as feeling bloated, pain, and discomfort in the abdomen, heartburn, and gas
- blood tests may indicate increased liver enzymes
- skin reactions after sun exposure or use of UVA lamps
- skin problems such as itching, irritation, or redness, dryness, rash
- muscle pain
- weakness or lack of energy
- chest pain
- sunburn.
Uncommon side effects(may affect up to 1 in 100 people):
- Low sodium levels in the blood. This can cause headache, dizziness, confusion, weakness, muscle cramps, or nausea and vomiting.
- blood test results may show decreased white blood cell count.
Reporting of side effects
If you experience any side effect, talk to your doctor or pharmacist, even if it is not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Esbriet
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the bottle, on the blister, and on the carton after EXP. The expiry date refers to the last day of the month stated.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Esbriet Composition
267 mg tablets
The active ingredient is pirfenidone. Each film-coated tablet contains 267 mg of pirfenidone.
The other ingredients are: microcrystalline cellulose, sodium croscarmellose (see section 2 "Esbriet contains sodium"), povidone K30, colloidal anhydrous silica, magnesium stearate.
The film coating consists of: polyvinyl alcohol, titanium dioxide (E171), macrogol 3350, talc, yellow iron oxide (E172).
534 mg tablets
The active ingredient is pirfenidone. Each film-coated tablet contains 534 mg of pirfenidone.
The other ingredients are: microcrystalline cellulose, sodium croscarmellose (see section 2 "Esbriet contains sodium"), povidone K30, colloidal anhydrous silica, magnesium stearate.
The film coating consists of: polyvinyl alcohol, titanium dioxide (E171), macrogol 3350, talc, yellow iron oxide (E172) and red iron oxide (E172).
801 mg tablets
The active ingredient is pirfenidone. Each film-coated tablet contains 801 mg of pirfenidone.
The other ingredients are: microcrystalline cellulose, sodium croscarmellose (see section 2 "Esbriet contains sodium"), povidone K30, colloidal anhydrous silica, magnesium stearate.
The film coating consists of: polyvinyl alcohol, titanium dioxide (E171), macrogol 3350, talc, red iron oxide (E172) and black iron oxide (E172).
Product Appearance and Package Contents
267 mg tablets
Esbriet 267 mg film-coated tablets are yellow, oval, biconvex, film-coated tablets with the inscription "PFD".
The bottle packs contain a bottle with 90 tablets, or two bottles with 90 tablets each (180 tablets in total).
The blister packs contain 21, 42, 84 or 168 film-coated tablets and the multipacks contain 63 film-coated tablets (2-week starter treatment 21 + 42) or 252 film-coated tablets (maintenance pack 3x84).
534 mg tablets
Esbriet 534 mg film-coated tablets are orange, oval, biconvex, film-coated tablets with the inscription "PFD".
The bottle packs contain a bottle with 21 tablets or a bottle with 90 tablets.
801 mg tablets
Esbriet 801 mg film-coated tablets are brown, oval, biconvex, film-coated tablets with the inscription "PFD".
The bottle pack contains a bottle with 90 tablets.
The blister packs contain 84 film-coated tablets and the multipack contains 252 film-coated tablets (maintenance pack 3x84).
Each of the 801 mg tablet blister strips is marked with the following symbols and abbreviations, as a reminder to take a dose three times a day:
 (dawn; morning dose)
(dawn; morning dose)  (sun; afternoon dose) and
(sun; afternoon dose) and  (moon; evening dose).
(moon; evening dose).
LU, MA, MI, JU, VI, SA, DO
Only some pack sizes may be marketed.
Marketing Authorization Holder
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Str. 1
D-79639 Grenzach-Wyhlen
Germany
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
|
Czech Republic Roche s.r.o. Tel: +420 - 2 20382111 | Hungary Roche (Hungary) Kft. Tel: +36 1 279 4500 |
Denmark Roche Pharmaceuticals A/S Tlf: +45 - 36 39 99 99 | Malta (See Ireland) |
Germany Roche Pharma AG Tel: +49 (0) 7624 140 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Estonia Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norway Roche Norge AS Tlf: +47 - 22 78 90 00 |
Greece Roche (Hellas) A.E. Τηλ: +30 210 61 66 100 | Austria Roche Austria GmbH Tel: +43 (0) 1 27739 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Poland Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Croatia Roche d.o.o. Tel: +385 1 4722 333 | Romania Roche România S.R.L. Tel: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenia Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovakia Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italy Roche S.p.A. Tel: +39 - 039 2471 | Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
Cyprus Γ.Α.Σταμ?της & Σια Λτδ. Τηλ: +357 - 22 76 62 76 | Sweden Roche AB Tel: +46 (0) 8 726 1200 |
Latvia Roche Latvija SIA Tel: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ESBRIET 267 mg FILM-COATED TABLETSDosage form: CAPSULE, 267 mg pirfenidoneActive substance: pirfenidoneManufacturer: Roche Registration GmbhPrescription requiredDosage form: CAPSULE, 267 mg pirfenidoneActive substance: pirfenidoneManufacturer: Roche Registration GmbhPrescription requiredDosage form: TABLET, 801 mgActive substance: pirfenidoneManufacturer: Roche Registration GmbhPrescription required
Online doctors for ESBRIET 267 mg FILM-COATED TABLETS
Discuss questions about ESBRIET 267 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions