ERELZI 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN

How to use ERELZI 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Erelzi 50mg solution for injection in pre-filled pen
etanercept
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- Your doctor will also give you a Patient Alert Card, which contains important safety information that you need to know before and during treatment with Erelzi.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you or the child in your care, do not pass it on to others, as it may harm them, even if their symptoms are the same as yours or the child in your care.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack:
- What is Erelzi and what is it used for
- What you need to know before you use Erelzi
- How to use Erelzi
- Possible side effects
- Storage of Erelzi
- Contents of the pack and other information
- Instructions for use of Erelzi in pre-filled pen
1. What is Erelzi and what is it used for
Erelzi is a medicine made from two human proteins. It blocks the activity of another protein in the body that causes inflammation. Erelzi works by reducing inflammation associated with certain diseases.
Erelzi can be used in adults aged 18 years and older for the treatment of moderate or severe rheumatoid arthritis, psoriatic arthritis, severe axial spondyloarthritis, including ankylosing spondylitis, and moderate or severe psoriasis, usually depending on each case, when other treatments have not been effective enough or are not suitable for you.
In the treatment of rheumatoid arthritis, Erelzi is usually used in combination with methotrexate, although it can also be used as a single medicine, in case methotrexate treatment is not suitable for you. Erelzi can slow down the damage caused by rheumatoid arthritis in your joints and improve your ability to perform daily activities, whether used alone or in combination with methotrexate.
In patients with psoriatic arthritis with multiple joint involvement, Erelzi can improve their ability to perform normal daily activities.
In patients with multiple symmetric joints that are swollen or painful (e.g., hands, wrists, and feet), Erelzi can delay the progression of structural damage to these joints caused by the disease.
Erelzi is also indicated for the treatment of children and adolescents with the following diseases:
- For the following types of juvenile idiopathic arthritis when treatment with methotrexate has not worked adequately, or is not suitable for them:
- Polyarthritis (with positive or negative rheumatoid factor) and extended oligoarthritis in patients aged 2 years and older and weighing 62.5 kg or more.
- Psoriatic arthritis in patients aged 12 years and older and weighing 62.5 kg or more.
- For enthesitis-related arthritis in patients aged 12 years and older and weighing 62.5 kg or more, for whom the use of other commonly used treatments has not worked adequately, or such treatments are not suitable for them.
- Severe psoriasis in patients aged 6 years and older and weighing 62.5 kg or more who have had an inadequate response to (or are unable to take) phototherapies or other systemic therapies.
2. What you need to know before you use Erelzi
Do not use Erelzi
- if you or the child in your care are allergic to etanercept or any of the other ingredients of Erelzi (listed in section 6). If you or the child experience allergic reactions, such as chest tightness, wheezing, dizziness, or rash, do not inject more Erelzi and contact your doctor immediately.
- if you or the child have or are at risk of developing a severe blood infection called sepsis. If you are not sure, consult your doctor.
- if you or the child have any type of infection. If you are not sure, consult your doctor.
Warnings and precautions
Consult your doctor before starting treatment with Erelzi.
- Allergic reactions: If you or the child experience allergic reactions such as chest tightness, wheezing, dizziness, or rash, do not inject more Erelzi and contact your doctor immediately.
- Infections/surgery: If you or the child develop a new infection or are about to undergo major surgery, your doctor may want to monitor your treatment with Erelzi.
- Infections/diabetes: Inform your doctor if you or the child have a history of recurrent infections or have diabetes or other disorders that increase the risk of infection.
- Infections/monitoring: Inform your doctor of any recent travel outside the European region. If you or the child develop symptoms of an infection such as fever, chills, or cough, notify your doctor immediately. Your doctor should decide whether to continue monitoring you or the child for the presence of infections after you or the child stop treatment with Erelzi.
- Tuberculosis: Since cases of tuberculosis have been reported in patients treated with Erelzi, your doctor will examine the signs and symptoms of tuberculosis before starting Erelzi. This may include a thorough medical history, chest X-ray, and tuberculosis test. The performance of these tests should be recorded on the Patient Alert Card. It is very important that you tell your doctor if you or the child have had tuberculosis or have been in close contact with someone who has had tuberculosis. If symptoms of tuberculosis (such as persistent cough, weight loss, apathy, moderate fever) or any other infection appear during or after treatment, inform your doctor immediately.
- Hepatitis B: Inform your doctor if you or the child have or have had hepatitis B. Your doctor should perform a hepatitis B test before you or the child start treatment with Erelzi. Treatment with Erelzi may reactivate hepatitis B in patients who have previously been infected with the hepatitis B virus. If this happens, you or the child should stop using Erelzi.
- Hepatitis C: Inform your doctor if you or the child have hepatitis C. Your doctor may want to monitor treatment with Erelzi in case the infection worsens.
- Blood disorders: Inform your doctor immediately if you or the child have signs or symptoms such as persistent fever, sore throat, bruising, bleeding, or paleness. Such symptoms may indicate a serious blood problem that requires interruption of treatment with Erelzi.
- Nervous system and vision disorders: Inform your doctor if you or the child have multiple sclerosis, optic neuritis (inflammation of the optic nerves), or transverse myelitis (inflammation of the spinal cord). Your doctor will decide if Erelzi is a suitable treatment.
- Congestive heart failure: Inform your doctor if you or the child have a history of congestive heart failure, as Erelzi needs to be used with caution in these circumstances.
- Cancer: Inform your doctor if you have or have had lymphoma (a type of blood cancer) or any other cancer before you are given Erelzi.
Patients with severe rheumatoid arthritis who have had the disease for a long time may be at a higher than average risk of developing lymphoma.
Children and adults taking Erelzi may have an increased risk of developing lymphoma or other cancers.
Some adolescent and child patients who have received Erelzi or other medicines that work in the same way as Erelzi have developed cancers, including unusual types, which sometimes resulted in death.
Some patients who receive Erelzi have developed skin cancers. Inform your doctor if you or the child develop any changes in the appearance of the skin or growths on the skin.
- Chickenpox: Inform your doctor if you or the child are exposed to chickenpox while using Erelzi. Your doctor will decide whether preventive treatment for chickenpox is appropriate.
- Alcoholism: Erelzi should not be used to treat alcohol-related hepatitis. Please inform your doctor if you or the child in your care have a history of alcoholism.
- Wegener's granulomatosis: Erelzi is not recommended for the treatment of Wegener's granulomatosis, a rare inflammatory disease. If you or the child in your care have Wegener's granulomatosis, discuss this with your doctor.
- Anti-diabetic medicines: Inform your doctor if you or the child have diabetes or are taking medicines to treat diabetes. Your doctor may decide if you or the child need less anti-diabetic medicine while using Erelzi.
Children and adolescents
The use of Erelzi is not indicated in children and adolescents weighing less than 62.5 kg.
- Vaccinations: If possible, children should have all vaccinations up to date before using Erelzi. Some vaccines, such as oral polio vaccine, should not be given while using Erelzi. Consult your doctor before you or the child use any vaccine.
Normally, Erelzi should not be used in children under 2 years of age or weighing less than 62.5 kg with polyarthritis or extended oligoarthritis, in children under 12 years of age or weighing less than 62.5 kg with enthesitis-related arthritis or psoriatic arthritis, or in children under 6 years of age or weighing less than 62.5 kg with psoriasis.
Other medicines and Erelzi
Inform your doctor or pharmacist if you or the child are using, have recently used, or might use any other medicine (including anakinra, abatacept, or sulfasalazine), even those not prescribed by your doctor.
You or the child must not useErelzi with medicines that contain the active substances anakinra or abatacept.
Pregnancy and breastfeeding
Erelzi should only be used during pregnancy if clearly necessary. Consult your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant.
If you have received Erelzi during pregnancy, your baby may be at a higher risk of infection. Additionally, in one study, more birth defects were observed when the mother had received etanercept during pregnancy, compared to mothers who had not received etanercept or other similar medicines (TNF antagonists), but there was no pattern in the types of birth defects reported. Another study did not find an increased risk of congenital malformations when the mother had received Erelzi during pregnancy. Your doctor will help you decide if the benefits of treatment outweigh the potential risk to your baby.
Consult your doctor if you wish to breastfeed while being treated with Erelzi. It is important that you inform the pediatrician and other healthcare professionals about the use of Erelzi during pregnancy and breastfeeding before your baby receives any vaccine.
Driving and using machines
Erelzi is not expected to affect your ability to drive and use machines.
Erelzi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 50 mg; this is essentially "sodium-free".
3. How to use Erelzi
Follow the instructions for administration of this medicine exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist again.
If you think the effect of Erelzi is too strong or too weak, talk to your doctor or pharmacist.
You have been prescribed a 50 mg presentation of Erelzi. A 25 mg presentation of Erelzi is available for 25 mg doses.
Use in adult patients (aged 18 years and older)
Rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis, including ankylosing spondylitis
The usual dose is 25 mg administered twice a week or 50 mg administered once a week, by subcutaneous injection. However, your doctor may decide on an alternative frequency for injecting Erelzi.
Psoriasis
The usual dose is 25 mg twice a week or 50 mg once a week.
Alternatively, 50 mg may be administered twice a week for a maximum of 12 weeks, followed by 25 mg twice a week or 50 mg once a week.
Your doctor will decide how long you should use Erelzi and whether you need to repeat treatment based on your response. If Erelzi has no effect on your disease after 12 weeks, your doctor may advise you to stop using this medicine.
Use in children and adolescents
The suitable dose and dosing frequency will depend on the child's or adolescent's body weight and disease. Your doctor will determine the suitable dose for the child and prescribe the most appropriate presentation of etanercept. Pediatric patients weighing 62.5 kg or more may be prescribed a dose of 25 mg twice a week or 50 mg once a week using a pre-filled syringe or fixed-dose pre-filled pen.
Other etanercept medicines with suitable doses for children are available.
For polyarthritis or extended oligoarthritis in patients aged 2 years and older and weighing 62.5 kg or more, or enthesitis-related arthritis or psoriatic arthritis in patients aged 12 years and older and weighing 62.5 kg or more, the usual dose is 25 mg twice a week or 50 mg once a week.
For psoriasis in patients aged 6 years and older and weighing 62.5 kg or more, the usual dose is 50 mg once a week. If Erelzi has no effect on the child's disease after 12 weeks, your doctor may advise you to stop using this medicine.
Your doctor will give you precise instructions for preparing and calculating the correct dose.
Form and route of administration
Erelzi is administered by subcutaneous injection.
In section 7, "Instructions for use of Erelzi in pre-filled pen", detailed instructions for injecting Erelzi are included.
The Erelzi solution should not be mixed with any other medicine.
To help you remember, it may be useful to note down in a diary which day(s) of the week you should use Erelzi.
If you use more Erelzi than you should
If you use more Erelzi than you should (either by injecting a high dose at one time or by using it more frequently), you should talk to a doctor or pharmacist immediately.
Always carry the medicine pack with you, even if it is empty.
If you forget to inject Erelzi
If you miss a dose, you should inject it as soon as you remember, unless the next dose is scheduled for the next day, in which case you should omit the missed dose. Then, continue injecting the medicine on the usual day(s). If you do not remember until the day you are due to receive the next dose, do not inject a double dose (two doses on the same day) to make up for the missed dose.
If you stop treatment with Erelzi
Your symptoms may return after stopping treatment.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Allergic Reactions
If you notice any of the following reactions, do not inject more Erelzi. Tell your doctor immediately or go to the Emergency Department of the nearest hospital.
- Difficulty swallowing or breathing.
- Swelling of the face, throat, hands, and feet.
- Feeling of nervousness or anxiety, palpitations, sudden reddening of the skin, and/or feeling of heat.
- Severe rash, itching, or hives (prominent red or pale skin rashes, often accompanied by itching).
Severe allergic reactions are rare. However, any of the above symptoms may be a sign of an allergic reaction to Erelzi, so you should seek immediate emergency medical attention.
Severe Adverse Effects
If you notice any of the following effects, you or the child may need urgent medical attention.
- Signs of severe infections, such as high fever that may be accompanied by cough, shortness of breath, chills, weakness, or a painful, sensitive, red, and hot area on the skin or joints.
- Signs of blood disorders, such as bleeding, bruising, or paleness.
- Signs of nervous system disorders, such as numbness or tingling, vision changes, eye pain, or weakness in an arm or leg.
- Signs of heart failure or worsening heart failure, such as fatigue or shortness of breath with activity, swelling of the ankles, feeling of fullness in the neck or abdomen, shortness of breath at night, or cough, blue discoloration of the nails or around the lips.
- Signs of cancer: cancer can affect any part of the body, including the skin and blood, and the possible signs will depend on the type and location of the cancer. These signs may include weight loss, fever, swelling (with or without pain), persistent cough, presence of lumps or thickening of the skin.
- Signs of autoimmune reactions (in which antibodies that can damage normal body tissues develop) such as pain, itching, weakness, and abnormal breathing, thinking, feeling, or vision.
- Signs of lupus or lupus-like syndrome, such as weight changes, persistent rash, fever, muscle or joint pain, or fatigue.
- Signs of blood vessel inflammation, such as pain, fever, redness, or heat of the skin, or itching.
These adverse effects are rare or infrequent, but they are serious conditions (some of which can be life-threatening). If these signs occur, tell your doctor immediately or go to the Emergency Department of the nearest hospital.
The following are the known adverse effects of Erelzi, grouped in decreasing order of frequency:
- Very common (may affect more than 1 in 10 people):
Infections (including colds, sinusitis, bronchitis, urinary tract infections, and skin infections); injection site reactions (including bleeding, bruising, redness, itching, pain, and swelling) do not occur as frequently after the first month of treatment; some patients have developed an injection site reaction that they had recently used.
- Common (may affect up to 1 in 10 people):
Allergic reactions; fever; rash; itching; antibodies directed against normal tissues (formation of autoantibodies).
- Uncommon (may affect up to 1 in 100 people):
Severe infections (including pneumonia, non-superficial skin infections, joint infections, blood infections, and generalized infections); worsening of congestive heart failure; low red blood cell count, low white blood cell count, low neutrophil count (a type of white blood cell); low platelet count; skin cancer (excluding melanoma); localized skin swelling (angioedema); hives (prominent red or pale skin rashes, often accompanied by itching); eye inflammation, psoriasis (new or worsening); inflammation of the blood vessels affecting multiple organs; increased liver enzymes in blood tests (in patients who also receive treatment with methotrexate, the increase in liver enzymes is common); abdominal cramps and pain, diarrhea, weight loss, or blood in the stool (signs of intestinal problems).
- Rare (may affect up to 1 in 1,000 people):
Severe allergic reactions (including severe localized skin swelling and wheezing); lymphoma (a type of blood cancer); leukemia (cancer that affects the blood and bone marrow); melanoma (a type of skin cancer); combined low red blood cell count, white blood cell count, and platelet count; nervous system disorders (with severe muscle weakness and symptoms similar to those of multiple sclerosis or inflammation of the optic nerves or spinal cord); tuberculosis; new-onset congestive heart failure; seizures; lupus or lupus-like syndrome (symptoms may include persistent rash, fever, joint pain, and fatigue); skin rash, which can lead to severe blistering and peeling of the skin; lichenoid reactions (itchy reddish-purple skin rash and/or thick white-gray lines on the mucous membranes); autoimmune hepatitis (in patients who also receive treatment with methotrexate, the frequency is uncommon); sarcoidosis (an immune disorder that can affect the lungs, skin, and lymph nodes); lung inflammation or scarring (in patients who also receive treatment with methotrexate, the frequency of lung inflammation or scarring is uncommon).
- Very rare (may affect up to 1 in 10,000 people):
Failure of the bone marrow to produce crucial blood cells.
- Frequency not known (cannot be estimated from the available data):
Merkel cell carcinoma (a type of skin cancer); excessive activation of white blood cells associated with inflammation (macrophage activation syndrome); reactivation of hepatitis B (a liver infection); damage to the small filters within the kidneys that lead to impaired kidney function (glomerulonephritis); worsening of a disease called dermatomyositis (inflammation and weakness of the muscles accompanied by skin rash).
Other Adverse Effects in Children and Adolescents
The adverse effects observed in children and adolescents, as well as their frequencies, are similar to those described above.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can help provide more information on the safety of this medicine.
5. Storage of Erelzi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and the label of the pre-filled pen after "EXP". The expiration date is the last day of the month indicated.
Store in a refrigerator (2 °C – 8 °C). Do not freeze.
Keep the pre-filled pens in the outer packaging to protect them from light.
After removing the pre-filled pen from the refrigerator, wait approximately 15-30 minutes for the Erelzi solution to reach room temperature. Do not heat it in any other way. Then, it is recommended to use it immediately.
Erelzi can be stored outside the refrigerator at a maximum temperature of 25 °C, and for a single period of up to 4 weeks; after which, the medicine cannot be refrigerated again. Erelzi should be discarded if it has not been used within 4 weeks of being removed from the refrigerator. It is recommended that you note the date on which Erelzi was removed from the refrigerator and the date from which Erelzi should be discarded (not later than 4 weeks from the removal of the packaging from the refrigerator).
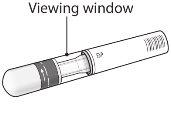
Inspect the solution in the pen by looking through the transparent inspection window. The solution should be clear or slightly opalescent, colorless to slightly yellowish, and may contain small white or almost translucent protein particles. This is the normal appearance of Erelzi. Do not use the solution if it is discolored or cloudy, or if it contains particles other than those described above. If you are concerned about the appearance of the solution, contact your pharmacist for any help you may need.
Medicines should not be thrown away via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container Contents and Additional Information
Erelzi Composition
The active ingredient is etanercept.
Each pre-filled pen contains 50 mg of etanercept.
The other components are anhydrous citric acid, sodium citrate dihydrate, sodium chloride, sucrose, L-lysine hydrochloride, sodium hydroxide, hydrochloric acid, and water for injectable preparations.
Product Appearance and Container Contents
Erelzi is presented as an injectable solution in a pre-filled pen. The pre-filled pen contains a clear or slightly opalescent injectable solution (injection), colorless to slightly yellowish. The pre-filled syringes are manufactured with Type I glass, a rubber stopper for the plunger (bromobutyl rubber), a plunger rod, a stainless steel needle of 29 gauge, and a needle cap (thermoplastic elastomer). Each container contains 1, 2, or 4 pens; multiple containers contain 12 pens (3 containers of 4 pens).
Only some package sizes may be marketed.
Marketing Authorization Holder
Sandoz GmbH
Biochemiestrasse 10
6250 Kundl
Austria
Manufacturer
Sandoz GmbH Schaftenau
Biochemiestrasse 10
6336 Langkampfen
Austria
Novartis Pharmaceutical Manufacturing GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Sandoz nv/sa Tel: +32 2 722 97 97 | Lithuania Sandoz Pharmaceuticals d.d filialas Tel: +370 5 2636 037 |
Bulgaria Sandoz Bulgaria EOOD Tel: +359 2 970 47 47 | Luxembourg Sandoz nv/sa Tel: +32 2 722 97 97 |
Czech Republic Sandoz s.r.o. Tel: +420 225 775 111 | Hungary Sandoz Hungária Kft. Tel: +36 1 430 2890 |
Denmark/Norway/Iceland/Sweden Sandoz A/S Tel: +45 63 95 10 00 | Malta Sandoz Pharmaceuticals d.d. Tel: +356 99644126 |
Germany Hexal AG Tel: +49 8024 908 0 | Netherlands Sandoz B.V. Tel: +31 36 52 41 600 |
Estonia Sandoz d.d. Eesti filiaal Tel: +372 665 2400 | Austria Sandoz GmbH Tel: +43 5338 2000 |
Greece SANDOZ HELLAS ΜΟΝΟΠΡΟΣΩΠΗ Α.Ε. Tel: +30 216 600 5000 | Poland Sandoz Polska Sp. z o.o. Tel: +48 22 209 70 00 |
Spain Sandoz Farmacéutica, S.A. Tel: +34 900 456 856 | Portugal Sandoz Farmacêutica Lda. Tel: +351 21 000 86 00 |
France Sandoz SAS Tel: +33 1 49 64 48 00 | Romania Sandoz Pharmaceuticals SRL Tel: +40 21 407 51 60 |
Croatia Sandoz d.o.o. Tel: +385 1 23 53 111 | Slovenia Sandoz farmacevtska družba d.d. Tel: +386 1 580 29 02 |
Ireland Rowex Ltd. Tel: + 353 27 50077 | Slovak Republic Sandoz d.d. - organizačná zložka Tel: +421 2 50 70 6111 |
Italy Sandoz S.p.A. Tel: +39 02 96541 | Finland Sandoz A/S Tel: +358 10 6133 400 |
Cyprus Sandoz Pharmaceuticals d.d. Tel: +357 22 69 0690 | United Kingdom (Northern Ireland) Sandoz GmbH Tel: +43 5338 2000 |
Latvia Sandoz d.d. Latvia filiale Tel: +371 67 892 006 |
Date of Last Revision of this Leaflet:{MM/AAAA}
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Instructions for Use of Erelzi Pre-filled Pen
| READ ALL INSTRUCTIONS BEFORE INJECTING THE MEDICINE.This same information is also available at www.erelzi.eu and in the following QR code. "Include QR code" + www.erelzi.eu These instructions will help you correctly administer Erelzi using the pre-filled SensoReady pen. It is essential that you do not attempt to inject the medicine until your doctor, nurse, or pharmacist has shown you how to do it. |
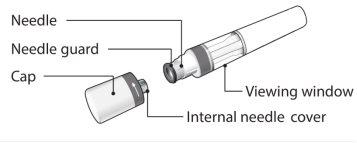
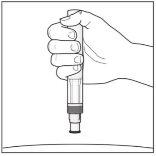
Your Erelzi Pre-filled Pen:
Erelzi pre-filled pen without cap. Do notremove the cap until you are ready to administer the injection. | Store the box with the pen in the refrigeratorbetween 2°C and 8°C and out of sight and reach of children.
To make the injection more comfortable, take the pen out of the refrigerator 15-30 minutesbefore use to allow it to reach room temperature. |
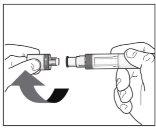
What You Need for the Injection:
Included in the box: New and unused Erelzi pre-filled pen. | Not included in the box:
|
|
|
Before the Injection:
|
The solution should be clear or slightly opalescent, colorless to slightly yellowish, and may contain small white or almost translucent protein particles. This is the normal appearance of Erelzi. Do not useif the liquid is cloudy, discolored, or contains large lumps, scales, or colored particles. Do not useif the pen has passed its expiration date. Do not useif the safety sealis broken. Contact your pharmacist if the pen does not meet any of these requirements. |
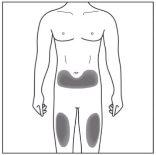
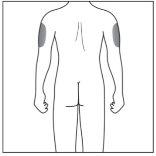
| 2a. Choose an Injection Site:
|
| 2b. Only for Caregivers or Healthcare Professionals:
|
|
|
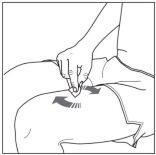
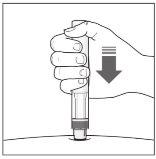
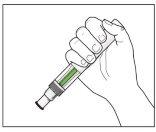
The Injection:
|
|
|
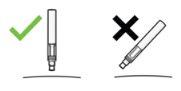
CorrectIncorrect |
| YOU MUST READ THE FOLLOWING BEFORE INJECTION. During the injection, you will hear 2loud clicks. The 1st clickindicates the start of the injection. After a few seconds, the 2nd clickwill indicate that the injection is about to finish. Keep the pen firmly pressed against the skin until the green indicatorfills the window and stops moving. |
|
|
|
|
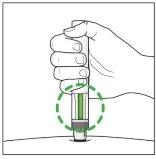
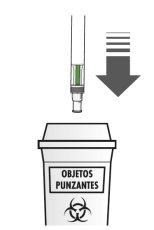
After the Injection:
|
|
|
|
If you have any questions, consult a doctor, nurse, or pharmacist who is familiar with Erelzi.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ERELZI 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PENDosage form: INJECTABLE, 25 mgActive substance: etanerceptManufacturer: Samsung Bioepis Nl B.V.Prescription requiredDosage form: INJECTABLE, 50 mgActive substance: etanerceptManufacturer: Samsung Bioepis Nl B.V.Prescription requiredDosage form: INJECTABLE, 50mgActive substance: etanerceptManufacturer: Samsung Bioepis Nl B.V.Prescription required
Online doctors for ERELZI 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN
Discuss questions about ERELZI 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions