ENTYVIO 108 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN

How to use ENTYVIO 108 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Entyvio 108mg injectable solution in pre-filled pen
vedolizumab
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Entyvio and what is it used for
- What you need to know before taking Entyvio
- How to take Entyvio
- Possible side effects
- Storage of Entyvio
- Package contents and additional information
1. What is Entyvio and what is it used for
What is Entyvio
Entyvio contains the active substance "vedolizumab". Vedolizumab belongs to a group of biological medicines called monoclonal antibodies (Mab).
How Entyvio works
Entyvio blocks a protein on the surface of white blood cells (leukocytes) that causes inflammation in ulcerative colitis and Crohn's disease, thereby reducing inflammation.
What Entyvio is used for
Entyvio is used to treat signs and symptoms in adults with:
- moderate to severe active ulcerative colitis
- moderate to severe active Crohn's disease.
Ulcerative colitis
Ulcerative colitis is a disease that causes inflammation of the large intestine. If you have ulcerative colitis, you will first be given other medications. If you do not respond satisfactorily or cannot tolerate these medications, your doctor may prescribe Entyvio to reduce the signs and symptoms of the disease.
Crohn's disease
Crohn's disease is a disease that causes inflammation of the digestive tract. If you have Crohn's disease, you will first be given other medications. If you do not respond satisfactorily or cannot tolerate these medications, your doctor may prescribe Entyvio to reduce the signs and symptoms of the disease.
2. What you need to know before taking Entyvio
Do not use Entyvio
- if you are allergic to vedolizumab or any of the other ingredients of this medicine (listed in section 6).
- if you have a severe active infection, such as tuberculosis, septicemia, severe vomiting or diarrhea (gastroenteritis), or infection of the nervous system.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before using Entyvio.
Tell your doctor, pharmacist, or nurse immediatelywhen you start using this medicine for the first time, during treatment, and between doses:
- if you experience double vision, blurred vision, or loss of vision, difficulty speaking, weakness in an arm or leg, a change in your gait, or balance problems, numbness, decreased or lost sensation, confusion, or memory loss. These symptoms could be signs of a serious and potentially life-threatening brain complicationknown as progressive multifocal leukoencephalopathy (PML).
- if you have an infection, or think you have an infection - the signs may include chills, shaking, persistent cough, or high fever. Some infections can be serious and even life-threatening if not treated.
- if you experience signs of an allergic reactionsuch as wheezing, difficulty breathing, hives, itching, swelling, or dizziness. For more detailed information, see the section on allergic reactions in section 4.
- if you are going to receive any vaccineor have recently received one. Entyvio may affect how you respond to a vaccine.
- if you have cancer, tell your doctor. Your doctor will have to decide whether it is possible to administer Entyvio.
- if you do not feel any better, as vedolizumab may take up to 14 weeks to work in some patients with very active Crohn's disease.
Children and adolescents
Entyvio is not recommended for use in children and adolescents (under 18 years of age) due to the lack of information on the use of this medicine in this age group.
Other medicines and Entyvio
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
- Entyvio should not be administered with other biological medicines that suppress the immune system, as the effects that may occur are unknown.
Tell your doctor if you have previously been administered:
- natalizumab (a medicine for multiple sclerosis) or
- rituximab (a medicine for certain types of cancer and rheumatoid arthritis).
Your doctor will have to decide whether it is possible to administer Entyvio.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Pregnancy
The effects of Entyvio on pregnant women are unknown. Therefore, this medicine is not recommended during pregnancy. You and your doctor must decide that the benefit to you is clearly greater than the potential risk to you and your baby.
If you are a woman of childbearing age, it is recommended that you avoid becoming pregnant during treatment with Entyvio. You should use adequate contraceptive methods during treatment and for at least 4.5 months after receiving the last dose.
Breastfeeding
Tell your doctor if you are breastfeeding or plan to breastfeed. Entyvio passes into breast milk. There is not enough information on the effects this may have on your baby and milk production. A decision must be made to either stop breastfeeding or stop treatment with Entyvio, taking into account the benefits of breastfeeding for your baby and the benefits of treatment for you.
Driving and using machines
The effects of this medicine on the ability to drive and use machines or tools are small. A small number of patients have felt dizzy after receiving Entyvio. If you feel dizzy, do not drive or use tools or machines.
Entyvio 108mg injectable solution contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
3. How to take Entyvio
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If in doubt, consult them again.
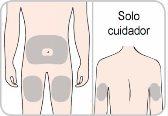
You or your caregiver will receive training on the use of subcutaneous injections (subcutaneous injections) of Entyvio.
How much Entyvio will be administered
Treatment with Entyvio is the same for ulcerative colitis and Crohn's disease.
The recommended dose is 108 mg of Entyvio administered by subcutaneous injection every 2 weeks.
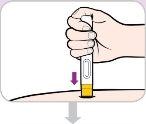
- At the start of treatment, your doctor will administer the initial doses of Entyvio through an intravenous infusion system in one of the arm veins (intravenous infusion) for about 30 minutes.
- After at least 2 intravenous infusions, you can start treatment with Entyvio by subcutaneous injection. The first subcutaneous injection is administered at the time of the next scheduled intravenous infusion, and every 2 weeks thereafter.
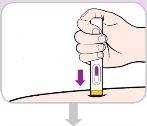
How to inject Entyvio
You can administer the subcutaneous injections yourself, or a caregiver can do so, after receiving training. At the end of this leaflet, you will find instructions on how to proceed with the subcutaneous injection.
If you miss or omit an Entyvio injection
If you miss or omit a dose, inject the next dose as soon as possible and then every 2 weeks.
If you stop treatment with Entyvio
Do not stop using Entyvio without talking to your doctor first.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Serious side effects
Tell your doctor immediatelyif you notice any of the following symptoms:
- allergic reactions (may affect up to 1 in 100 patients), with signs such as wheezing or difficulty breathing, hives, itching, swelling, or feeling unwell, pain at the infusion site, redness of the skin
- infections (may affect up to 1 in 10 patients), with signs such as chills or shaking, high fever, or rash
Other side effects
Tell your doctor as soon as possibleif you notice any of the following symptoms:
Very common side effects(may affect more than 1 in 10 patients)
- common cold
- joint pain
- headache
Common side effects(may affect up to 1 in 10 patients)
- pneumonia
- bacterial infection of the colon by Clostridium difficile
- fever
- respiratory infection
- changes in liver function, increased liver enzymes (shown in blood tests)
- fatigue
- cough
- flu
- back pain
- sore throat
- sinusitis
- itching/pruritus
- rash and redness
- limb pain
- muscle cramps
- muscle weakness
- throat infection
- stomach flu
- anal infection
- anal pain
- hard stools
- bloating
- gas
- high blood pressure
- numbness or tingling
- heartburn
- hemorrhoids
- stuffy nose
- eczema
- night sweats
- acne (pimples)
- reactions at the injection site (pain, swelling, redness, or itching)
- shingles
Uncommon side effects(may affect up to 1 in 100 patients)
- redness and sensitivity of the hair follicles
- infection of the mouth and throat by yeast
- vaginal infection
- blurred vision (loss of visual acuity)
Rare side effects(may affect up to 1 in 10,000 patients)
- sudden and severe allergic reaction that can cause difficulty breathing, inflammation, rapid heartbeat, sweating, low blood pressure, dizziness, loss of consciousness, and fainting (anaphylactic reaction and anaphylactic shock)
- inflammation of the liver (hepatitis). The signs and symptoms of hepatitis may include abnormal liver function tests, yellowing of the eyes or skin (jaundice), pain in the right upper abdomen, or bruising
Frequency not known(frequency cannot be estimated from available data)
- lung disease that causes difficulty breathing (interstitial lung disease)
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Entyvio
- Keep this medicine out of the sight and reach of children.
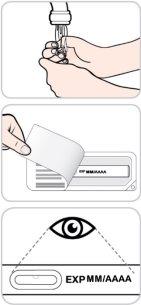
- Do not use this medicine after the expiration date stated on the carton and label, after "EXP". The expiration date is the last day of the month indicated.
- Entyvio is for single use.
- Store in a refrigerator (between 2°C and 8°C). Keep the pre-filled pen(s) in the original carton to protect them from light. If necessary, a single pre-filled pen can be stored outside the refrigerator, protected from light, at room temperature (up to 25°C) for 7 days, maximum. Do not use the pen if it has been outside the refrigerator for more than 7 days.
- Do not freeze. Do not expose directly to sunlight.
- Do not use this medicine if you notice particles in the liquid or discoloration (should be colorless or have a yellowish tint) before administration.
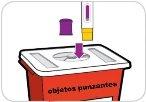
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Entyvio
- The active substanceis vedolizumab. Each pre-filled pen contains 108 mg of vedolizumab.
- The other componentsare citric acid monohydrate, sodium citrate dihydrate, L-histidine, L-histidine monohydrochloride, L-arginine hydrochloride, polysorbate 80, and water for injectable preparations.
Appearance and Container Contents of the Product
- Entyvio is a colorless or yellowish injectable solution provided in a glass pre-filled pen with an automatic safety mechanism that protects and locks the needle after removing the device from the injection site.
- Entyvio is available in packs containing 1 or 2 pre-filled pens and in multipacks containing 6 (6 x 1) pre-filled pens. Not all pack sizes may be marketed.
Marketing Authorization Holder
Takeda Pharma A/S
Delta Park 45
2665 Vallensbaek Strand
Denmark
Manufacturer
Takeda Austria GmbH
St. Peter-Straße 25
A-4020 Linz
Austria
You can request more information about this medicinal product from the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Takeda Belgium NV Tel.: +32 2 464 06 11 | Lithuania Takeda, UAB Tel.: +370 521 09 070 |
| Luxembourg/Luxemburg Takeda Belgium NV Tel.: +32 2 464 06 11 |
Czech Republic Takeda Pharmaceuticals Czech Republic s.r.o. Tel: +420 234 722 722 | Hungary Takeda Pharma Kft. Tel.: +36 1 270 7030 |
Denmark Takeda Pharma A/S Tlf: +45 46 77 10 10 | Malta Drugsales Ltd Tel.: +356 2141 9070 |
Germany Takeda GmbH Tel.: +49 (0) 800 825 3325 | Netherlands Takeda Nederland B.V. Tel.: +31 20 203 5492 |
Estonia Takeda Pharma OÜ Tel.: +372 6177 669 | Norway Takeda AS Tlf.: +47 800 800 30 |
Greece TAKEDA ΕΛΛΑΣ Α.Ε. Τηλ.: +30 210 6387800 | Austria Takeda Pharma Ges.m.b.H. Tel.: +43 (0) 800 20 80 50 |
Spain Takeda Farmacéutica España, S.A. Tel.: +34 917 90 42 22 | Poland Takeda Pharma Sp. z o.o. Tel.: +48 223 062 447 |
France Takeda France SAS Tel.: +33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel.: +351 21 120 1457 |
Croatia Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | Romania Takeda Pharmaceuticals SRL Tel.: +40 21 335 03 91 |
Ireland Takeda Products Ireland Ltd. Tel.: 1800 937 970 | Slovenia Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel.: +386 (0) 59 082 480 |
Iceland Vistor hf. Simi: +354 535 7000 | Slovakia Takeda Pharmaceuticals Slovakia s.r.o. Tel.: +421 (2) 20 602 600 |
Italy Takeda Italia S.p.A Tel.: +39 06 502601 | Finland Takeda Oy Puh./Tel.: 0800 774 051 |
Cyprus A.POTAMITIS MEDICARE LTD Τηλ: +357 22583333 | Sweden Takeda Pharma AB Tel.: 020 795 079 |
Latvia Takeda Latvia SIA Tel.: +371 67840082 | United Kingdom (Northern Ireland) Takeda UK Ltd Tel.: +44 (0) 3333 000 181 |
Date of last revision of this leaflet: 03/2025
Other sources of information
This leaflet is available in a format suitable for blind or partially sighted patients and can be requested from the local representative of the marketing authorization holder.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
-----------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the administered product should be clearly recorded.
Instructions for use:
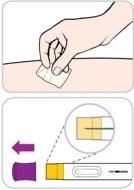
Read and follow these instructions before proceeding with the injection. Your doctor, nurse, or pharmacist should show you how to use the Entyvio pre-filled pen before using it for the first time.
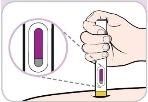
Your single-use Entyvio pre-filled pen
Before use | |||
Purple cap | Viewing window | ||
|
After use | |||
Yellow needle protection | Viewing window (injection completed) | ||
|
| |
| Wait 30minutes
|
| |
|
|
| |
|
|
| |
| PRESS
HOLD (count to 10)
CONFIRM
|
| |
|
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ENTYVIO 108 mg SOLUTION FOR INJECTION IN PRE-FILLED PENDosage form: INJECTABLE, 108 mgActive substance: vedolizumabManufacturer: Takeda Pharma A/SPrescription requiredDosage form: INJECTABLE INFUSION, 300 mgActive substance: vedolizumabManufacturer: Takeda Pharma A/SPrescription requiredDosage form: INJECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for ENTYVIO 108 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN
Discuss questions about ENTYVIO 108 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions