ENGERIX-B JUNIOR 10 micrograms/0.5 ml, PRE-FILLED SYRINGE SUSPENSION


How to use ENGERIX-B JUNIOR 10 micrograms/0.5 ml, PRE-FILLED SYRINGE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Engerix-B Junior 10 micrograms/0.5 ml suspension for injection in pre-filled syringe
Hepatitis B vaccine (rDNA, adsorbed) (HBV)
Read all of this leaflet carefully before you or your child start receiving this vaccine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed for you or your child, do not pass it on to others.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Throughout this leaflet, any reference to “you” also means “your child”.
Contents of the package leaflet
- What is Engerix-B Junior and what is it used for
- What you need to know before you receive Engerix-B Junior
- How Engerix-B Junior is administered
- Possible side effects
- Storage of Engerix-B Junior
- Contents of the pack and further information
1. What is Engerix-B Junior and what is it used for
Engerix-B Junior is a vaccine that is used to protect against hepatitis B infection. It may also help protect against hepatitis D infection.
This vaccine can be given to newborns, children, and adolescents up to 15 years of age.
Hepatitis B is an infectious disease of the liver caused by a virus. Some people have the hepatitis B virus in their body but cannot get rid of it. These people can continue to infect others and are known as carriers. The disease spreads when the virus enters the body after contact with bodily fluids, almost always blood, from an infected person.
If a mother is a carrier of the virus, she can pass the virus to her child during birth. It is also possible to contract the virus from a carrier through, for example, unprotected sex, sharing injectable syringes, or treatment with medical equipment that has not been properly sterilized.
The main signs of the disease include headache, fever, malaise (nausea), and jaundice (the skin and eyes turn yellow), although in about three out of 10 patients, there are no signs of disease. Of those infected with hepatitis B, one in 10 adults and up to nine in 10 children will become carriers of the virus and are likely to develop severe liver damage and, in some cases, liver cancer.
How Engerix-B Junior works
Engerix-B Junior contains a small amount of the outer surface of the hepatitis B virus. This outer surface is not infectious and cannot cause the disease.
- When you receive the vaccine, it will activate your body's immune system to prepare it to protect itself against these viruses in the future.
- Engerix-B Junior will not protect you if you have already contracted the hepatitis B virus.
- Engerix-B Junior can only help protect you against hepatitis B virus infection.
2. What you need to know before you receive Engerix-B Junior
Do not receive Engerix-B Junior:
- If you are allergic (hypersensitive) to Engerix-B Junior or any of the other components of this vaccine (listed in section 6).
- If you have a fever (high body temperature).
Engerix-B Junior should not be administered if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist before receiving Engerix-B Junior. Inform your doctor or pharmacist if you have any allergies or if you have ever had health problems after receiving a vaccine.
Warnings and precautions
Talk to your doctor or pharmacist before receiving Engerix-B Junior if you:
- Are on dialysis for a kidney problem or have a disease that may affect your immune system.
People who need dialysis, have chronic liver problems, are hepatitis C carriers, or are HIV-positive can also receive Engerix-B Junior. This is because hepatitis B infections can be severe in these patients. You will find more information about kidney problems and dialysis in section 3.
If you are not sure if any of the above applies to you, talk to your doctor before receiving Engerix-B Junior.
Fainting (especially in adolescents) may occur before or after any injection, so you should inform your doctor or nurse if you have fainted after receiving an injection in the past.
Like other vaccines, Engerix-B Junior may not completely protect you against hepatitis B. A number of factors such as advanced age, sex, overweight, smoking, and some chronic problems reduce the immune response to the vaccine. If any of these apply to you, your doctor may decide to perform a blood test or administer additional doses of Engerix-B Junior to ensure you are protected.
Using Engerix-B Junior with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Engerix-B Junior can be administered at the same time as most routine vaccines. Your doctor will ensure that the vaccines are injected separately and in different parts of the body.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
It is unlikely that Engerix-B Junior will affect your ability to drive or use machines. However, do not drive or use machines if you feel unwell.
Engerix-B Junior contains sodium
This medicine contains less than 23 mg (1 mmol) of sodium per dose, so it is essentially “sodium-free”.
3. How Engerix-B Junior is administered
How your vaccine is administered
Your doctor will administer the recommended dose of Engerix-B Junior.
Engerix-B Junior will be administered:
- as an injection into the muscle of the upper arm in children and adolescents
- as an injection into the thigh in newborns and young children
- as an injection under the skin if you bruise easily or have bleeding problems.
How much is administered
You will receive a series of Engerix-B Junior injections. Once you have completed the injection schedule, you can expect long-term protection against hepatitis B.
- Newborns, children, and adolescents up to 15 years of age will normally receive the 10 micrograms/0.5 ml vaccine.
There are several alternative administration schedules for Engerix-B Junior. Your doctor will choose the most suitable schedule for you.
Schedule 1 – for newborns, children, and adolescents up to 15 years of age
First injection - now
Second injection - 1 month after the first injection
Third injection - 6 months after the first injection
Schedule 2 – for newborns, children, and adolescents up to 15 years of age
First injection - now
Second injection - 1 month after the first injection
Third injection - 2 months after the first injection
Fourth injection - 12 months after the first injection
- In newborns, Schedule 2 allows Engerix-B Junior to be administered at the same time as other routine childhood vaccines.
- This schedule can also be used if you are being vaccinated due to recent exposure to hepatitis B, as it will provide faster protection.
It is very important that you receive your injections at the recommended intervals. If you have any questions about the amount of vaccine you will receive, talk to your doctor.
Vaccination and childbirth
If you have hepatitis B and have just given birth, you can use Schedule 1 or 2 to vaccinate your child.
- Your doctor will also decide whether to administer hepatitis B immunoglobulin (human antibodies) to your child at the time of the first injection. This will help protect your child from hepatitis B. It will be administered in a different injection site.
Kidney problems and dialysis
If your child has a kidney problem or is on dialysis, your doctor may decide to perform a blood test or administer additional doses of the vaccine to ensure your child is protected.
4. Possible side effects
Like all vaccines, this vaccine can cause side effects, although not everybody gets them. The following side effects may occur with this vaccine:
Allergic reactions
If you have an allergic reaction, see a doctor immediately. The signs may include:
- swelling of the face
- low blood pressure
- difficulty breathing
- the skin turns blue
- loss of consciousness.
These signs usually start very soon after the injection is administered. See a doctor immediately if they occur after you leave the clinic.
Other side effects include:
Very common(these may occur in more than 1 in 10 doses of the vaccine): headache, pain and redness at the injection site, feeling tired, irritability.
Common(these may occur in up to 1 in 10 doses of the vaccine): numbness, nausea or vomiting, diarrhea or abdominal pain, loss of appetite, fever (high body temperature), feeling unwell, swelling at the injection site, reactions at the injection site, such as induration.
Uncommon(these may occur in up to 1 in 100 doses of the vaccine): dizziness, muscle pain, flu-like symptoms.
Rare(these may occur in up to 1 in 1,000 doses of the vaccine): swollen glands, hives, skin rash and itching, joint pain, tingling.
Side effects reported during the marketing of Engerix-B Junior are: easy bruising and inability to stop bleeding if you cut yourself, low blood pressure, inflammation of blood vessels, sudden swelling of the face around the mouth and throat area (angioneurotic edema), inability to move muscles (paralysis), inflammation of nerves (neuritis) that can cause loss of sensation or numbness, including temporary inflammation of nerves, causing pain, weakness, and paralysis in the limbs and often progressing to the chest and face (Guillain-Barré syndrome), a disease of the nerves of the eye (optic neuritis) and multiple sclerosis, problems moving arms or legs (neuropathy), inflammation of the brain (encephalitis), degenerative brain disease (encephalopathy), infection around the brain (meningitis), seizures (convulsions), loss of skin sensation to pain or touch (hypoesthesia), purple or purple-red spots on the skin (lichen planus), red or purple spots on the skin, pain and stiffness in the joints (arthritis), muscle weakness.
In very premature newborns (at 28 weeks of gestation or earlier), the time between breaths may be longer than normal during the 2-3 days following vaccination.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's online platform, https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Engerix-B Junior
- Keep this vaccine out of the sight and reach of children.
- Do not use this vaccine after the expiry date which is stated on the label and carton, after EXP. The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze.
- Store in the original package to protect from light.
- Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicine to a pharmacy for disposal. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Engerix-B Junior
- The active substance is the outer surface of the hepatitis B virus. Each dose contains 10 micrograms/0.5 ml of protein composed of this outer surface, adsorbed onto hydrated aluminum hydroxide.
- The other ingredients are sodium chloride, disodium phosphate dihydrate, dibasic sodium phosphate, and water for injections.
Appearance and pack contents
Engerix-B Junior is a white, turbid liquid.
Engerix-B Junior (10 micrograms/0.5 ml) is available in a pre-filled syringe with or without a separate needle; pack sizes of 1 and 10.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel.: +34 900 202 700
Manufacturer
GlaxoSmithKline Biologicals s.a., Rue de l’Institut 89, B-1330 Rixensart, Belgium
or
SmithKline Beecham, S.A., Carretera de Ajalvir. Km. 2,5.- 28806 Alcalá de Henares. Madrid
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria, Denmark, Finland, Iceland, Norway, Sweden: Engerix-B
Belgium, Luxembourg: Engerix B Junior
France, Ireland, Italy: Engerix B-10
Germany: Engerix-B Children
Greece: Engerix
Spain, Netherlands: Engerix-B Junior
Portugal: Engerix B
Date of last revision of this leaflet:02/2024
Other sources of information
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
---------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Once stored, the content may present as a fine white deposit with a clear colorless supernatant. After shaking, the vaccine is slightly opaque.
Before using the vaccine, it should be visually inspected for foreign particles and/or abnormal physical appearance. Do not administer the vaccine if you observe either of these.
All the contents of the monodose container should be withdrawn and used immediately after withdrawal.
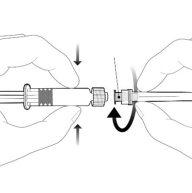
Instructions for the pre-filled syringe
| Hold the syringe by the body, not by the plunger. Remove the syringe cap by twisting it counterclockwise. |
| To insert the needle, attach the base to the luer-lock adapter and twist it a quarter turn clockwise until you feel it click. Do not pull the plunger out of the syringe body. If this happens, do not administer the vaccine. |
Disposal of waste
Disposal of unused medicine and all materials that have come into contact with it should be done in accordance with local regulations.
- Country of registration
- Average pharmacy price10.27 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ENGERIX-B JUNIOR 10 micrograms/0.5 ml, PRE-FILLED SYRINGE SUSPENSIONDosage form: INJECTABLE, 20 mcg Hepatitis B Surface Antigen/ mlActive substance: hepatitis B, purified antigenManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: INJECTABLE, 20 µgActive substance: hepatitis B, purified antigenManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 20 µgActive substance: hepatitis B, purified antigenManufacturer: Glaxosmithkline BiologicalsPrescription required
Online doctors for ENGERIX-B JUNIOR 10 micrograms/0.5 ml, PRE-FILLED SYRINGE SUSPENSION
Discuss questions about ENGERIX-B JUNIOR 10 micrograms/0.5 ml, PRE-FILLED SYRINGE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions