
Engerix B

Ask a doctor about a prescription for Engerix B

How to use Engerix B
Leaflet accompanying the packaging: patient information
Engerix B 20 micrograms – suspension for injection
Vaccine against hepatitis B virus infection (rDNA)
Hepatitis B vaccine (rDNA)
Read the leaflet carefully before using the vaccine because
it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed specifically for you. Do not pass it on to others.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Engerix B vaccine and what is it used for
- 2. Important information before using Engerix B vaccine
- 3. How to use Engerix B vaccine
- 4. Possible side effects
- 5. How to store Engerix B vaccine
- 6. Contents of the pack and other information
1. What is Engerix B vaccine and what is it used for
Engerix B is a vaccine used to prevent hepatitis B virus infection
(hepatitis B).
Engerix B 20 micrograms (adult dose) is indicated for use in adolescents from 16 years of age and adults.
Engerix B 20 micrograms (adult dose) can also be used in adolescents from 11 to 15 years of age, provided that the risk of hepatitis B virus infection during the vaccination cycle is low and it is certain that the 2-dose vaccination cycle will be completed. If these conditions cannot be met (e.g., in patients undergoing hemodialysis, people traveling to areas where hepatitis B is common, people in close contact with infected individuals), the standard Engerix B 10 micrograms (child dose) vaccination should be used.
The vaccine causes the body to produce its own immunity by producing antibodies against the hepatitis B virus.
Hepatitis B virus infection (hepatitis B): This disease is caused by the hepatitis B virus, which causes inflammatory liver swelling. The virus is found in body fluids such as blood, semen, vaginal discharge, saliva (sputum) of an infected person.
The disease may be asymptomatic for a period of 6 weeks to 6 months from infection.
Some infected people may not have any symptoms or may have mild flu-like symptoms.
Usually, infected people feel general malaise and fatigue. They may experience nausea, vomiting, dark urine, pale face, yellow skin and/or eyes, and other symptoms that may require hospital treatment.
Most adults fully recover, but some people, especially children, despite no symptoms of the disease, remain infected. These people are carriers of the hepatitis B virus. Carriers of the virus can infect other people around them and are at risk of serious liver diseases such as liver cirrhosis and liver cancer.
Vaccination is the best way to prevent the disease. None of the vaccine components are infectious.
2. Important information before using Engerix B vaccine
When not to use Engerix B vaccine
- if the patient is allergic to the active substance or any of the other ingredients of this vaccine (listed in section 6). Allergic reactions include itchy skin rash, difficulty breathing, face or tongue swelling;
- if the patient has a high fever (body temperature above 38.0 ° C). A mild infection, such as a cold, should not be a contraindication to vaccination, but you should tell your doctor about it.
Warnings and precautions
Tell your doctor if the patient:
- has previously had health problems after vaccinations, especially against hepatitis B virus infection (including allergic reactions);
- has bleeding problems or bruises easily.
Fainting (especially in adolescents) may occur after or even before injection of each vaccine dose. Therefore, you should inform your doctor or nurse if the child has ever fainted during injection.
Using Engerix B vaccine in patients with chronic liver disease or impaired immunity:
Patients with chronic liver disease, carriers of hepatitis C virus, can be vaccinated against hepatitis B virus infection.
Engerix B can also be used in people with impaired immunity, including those infected with HIV and patients with renal failure, including those undergoing hemodialysis. It should be considered that they may not develop sufficient protection after a full vaccination cycle and may require additional doses of the vaccine.
It has also been observed that a number of factors can affect the reduced efficacy of hepatitis B vaccination. These include: older age, male sex, obesity, smoking, route of vaccine administration, and some chronic diseases. In such cases, it may also be necessary to administer additional doses of the vaccine.
In all these cases, the doctor will decide on the possible need for additional blood tests and the proper timing and method of vaccination.
As with other vaccines, it is possible that not all vaccinated individuals will develop a protective immune response.
Engerix B vaccine and other medicines
Tell your doctor about all medicines taken recently and vaccinations.
Engerix B can be given at the same time as vaccines against tuberculosis, hepatitis A virus infection, polio, measles, mumps, rubella, diphtheria, tetanus, but the injection sites must be different.
Concomitant administration of Engerix B vaccine and standard dose of hepatitis B immune globulin at different sites does not affect the level of antibodies produced.
Engerix B vaccine can be given at the same time as the human papillomavirus (HPV) vaccine. Concomitant administration of Engerix B vaccine with Cervarix (HPV vaccine) did not show a clinically significant effect on antibody production against HPV antigens.
Pregnancy, breastfeeding, and fertility
Before using any medicine during pregnancy or breastfeeding, consult your doctor.
The effect of vaccine administration on fetal development has not been evaluated. It is believed that, like other inactivated viral vaccines, Engerix B does not pose a significant risk to the fetus.
Engerix B can be given to a pregnant woman only if there are clear indications for immunization and the benefits to the mother outweigh the potential risk to the fetus.
Clinical trials have not studied the effect of Engerix B vaccine administered to mothers on breastfed children. There is also no information on the excretion of hepatitis B virus antigen contained in the vaccine with milk. There is no contraindication to vaccinating breastfeeding mothers.
The doctor should discuss the benefits and potential risks associated with Engerix B vaccination during pregnancy and breastfeeding.
Driving and using machines
It is unlikely that Engerix B vaccine will have a significant effect on the ability to drive and use machines. However, if the patient feels unwell, they should not drive or operate machinery.
Other information
Due to the long incubation period of hepatitis B virus infection, it may happen that the vaccine is administered during the incubation period of the disease. In such cases, the vaccine may not prevent the development of infection.
Administration of Engerix B vaccine does not prevent liver infections caused by other pathogens.
This vaccine contains less than 1 mmol (23 mg) of sodium per dose, i.e., the vaccine is considered "sodium-free".
3. How to use Engerix B vaccine
Engerix B vaccine should be injected intramuscularly into the upper arm.
Do not administer Engerix B vaccine into the buttock muscle or subcutaneously, as this may not provide sufficient protection.
Exceptionally, in patients with bleeding disorders, due to the risk of bleeding after intramuscular administration, subcutaneous administration of the vaccine is allowed.
The vaccine should never be administered intravenously.
The following primary vaccination schedules are recommended:
The 3-dose schedule (0, 1, 6 months) results in slower development of optimal immune response, but provides a higher level of antibodies against hepatitis B virus.
- First dose: at any time
- Second dose: 1 month later
- Third dose: 6 months after the first dose
The accelerated schedule, when doses are given according to the 0, 1, 2 months schedule, allows for rapid development of optimal immune response and provides better cooperation from the vaccinated person. It is recommended to administer a fourth dose 12 months after the first dose.
- First dose: at any time
- Second dose: 1 month later
- Third dose: 2 months after the first dose
- Fourth dose: 12 months after the first dose
In exceptional cases, in adults over 18 years of age, e.g., before travel, when rapid primary vaccination is required within one month before departure and the 0, 1, 6 months schedule cannot be used, three doses of the vaccine can be administered on days 0, 7, 21. In the case of such a schedule, it is recommended to administer a fourth dose 12 months after the first dose.
- First dose: at any time
- Second dose: 7 days later
- Third dose: 21 days after the first dose
- Fourth dose: 12 months after the first dose
Engerix B 20 micrograms (adult dose) can also be used in children from 11 to 15 years of age, according to a 2-dose vaccination schedule (0, 6 months), provided that the risk of hepatitis B virus infection during the vaccination cycle is low and it is certain that the vaccination cycle will be completed.
- First dose: at any time
- Second dose: 6 months after the first dose
If these conditions cannot be met (e.g., patients undergoing hemodialysis, people traveling to areas where hepatitis B is common, people in close contact with infected individuals), the standard Engerix B 10 micrograms (child dose) vaccination should be used.
Make sure that the full vaccination course consisting of three (or two) or four doses of the vaccine has been administered. If not all doses have been administered, the vaccinated person may not be fully protected against the disease.
The doctor should inform about the possible need for additional doses and booster doses.
If the next dose of the vaccine has not been administered on time, it is necessary to talk to the doctor to schedule the next visit.
4. Possible side effects
Like all medicines, Engerix B vaccine can cause side effects, although not everybody gets them.
The following side effects have been reported after vaccination:
♦ Very common (may occur in 1 in 10 doses of the vaccine or more often):
- pain and redness at the injection site, feeling of tiredness
- irritability
♦ Common (less common than 1 in 10 doses of the vaccine):
- headache
- drowsiness
- nausea, vomiting, diarrhea, abdominal pain
- decreased appetite
- fever (37.5°C or higher), malaise, swelling at the injection site, other reactions at the injection site (such as induration)
♦ Uncommon (less common than 1 in 100 doses of the vaccine):
- dizziness
- muscle pain
- flu-like symptoms
♦ Rare (less common than 1 in 1,000 doses of the vaccine):
- generalized lymph node enlargement
- paresthesia (tingling sensation)
- hives, itching, rash
- joint pain
♦ Very rare (less common than 1 in 10,000 doses of the vaccine):
Side effects reported after the vaccine was placed on the market:
- thrombocytopenia (bleeding or easier than usual bruising due to a decrease in platelet count)
- encephalitis, encephalopathy, seizures, paralysis, neuritis, neuropathy, sensory impairment
- erythema multiforme, angioedema, lichen planus (skin and subcutaneous tissue disorders)
- arthritis, muscle weakness
- meningitis
- vasculitis, decreased blood pressure
- allergic reactions, including anaphylactic and anaphylactoid reactions, and reactions similar to serum sickness. They can occur as a local or generalized rash (which can be itchy or blistering), swelling of the eyelids and face, difficulty breathing or swallowing, sudden drop in blood pressure, or loss of consciousness. These reactions may occur even before leaving the doctor's office. In any case, immediate medical attention should be sought.
If the above events do not resolve or worsen, you should inform your doctor.
If you experience any other side effects not listed in this leaflet, please inform your doctor.
Do not be concerned by this list of possible side effects. It is possible that no side effects will occur after vaccination.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C
02-222 Warsaw
Phone: 22 49 21 301
Fax: 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Engerix B vaccine
Store in a refrigerator (2°C – 8°C).
Do not freeze.
The vaccine should be stored out of sight and reach of children.
Store the vaccine in its original packaging to protect it from light.
Do not use this vaccine after the expiry date stated on the packaging after "EXP". The expiry date refers to the last day of the specified month.
The "Lot" abbreviation means the product batch number.
6. Contents of the pack and other information
What Engerix B vaccine contains
- The active substance of Engerix B vaccine is:
Hepatitis B surface antigen (HBsAg)
20 micrograms
adsorbed onto aluminum hydroxide, hydrated
total: 0.50 milligrams Al
obtained from yeast cell culture (Saccharomyces cerevisiae) using DNA recombination technology
- The other ingredients are: sodium chloride, disodium phosphate dihydrate, sodium dihydrogen phosphate dihydrate, water for injections.
What Engerix B vaccine looks like and what the pack contains
Engerix B (20 micrograms in 1 ml) is a suspension for injection in a vial or pre-filled syringe.
Packaging available:
- 1-dose pre-filled syringes, packs of 1, 10, or 25,
- 1-dose vials, packs of 1, 10, or 100, with or without a syringe.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
GlaxoSmithKline Biologicals S.A.
rue de l’Institut 89
1330 Rixensart, Belgium
To obtain more detailed information, please contact your local representative of the marketing authorization holder:
GSK Services Sp. z o.o.
ul. Rzymowskiego 53
02-697 Warsaw
phone: (22) 576-90-00
Date of last revision of the leaflet:April 2023
-------------------------------------------------------------------------------------------------------------------------
Information intended only for healthcare professionals:
During storage, a white precipitate and a clear colorless liquid may form above.
Immediately before administration, the vaccine should be shaken vigorously to obtain a slightly cloudy suspension and inspected for any particles or changes in appearance. If any of these abnormalities are found, the vaccine should not be administered.
Instructions for the pre-filled syringe
Hold the pre-filled syringe by the body, not by the plunger.
Remove the cap from the pre-filled syringe by twisting it in the opposite direction to the clockwise direction.
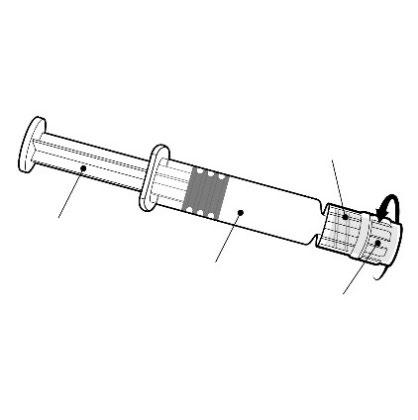
Luer Lock adapter
Plunger
Body
Cap
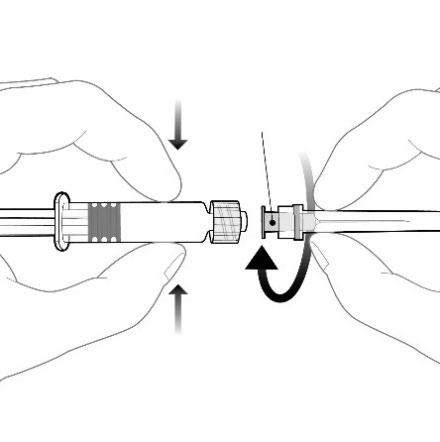
Attach the needle to the pre-filled syringe by connecting the needle hub to the Luer Lock adapter (Luer Lock Adaptor, LLA) and twisting it a quarter turn in the clockwise direction until the needle is locked.
Do not pull the plunger out of the pre-filled syringe body. If this happens, do not administer the vaccine.
Disposal
Any unused product or waste material should be disposed of in accordance with local regulations.
Needle cap
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGlaxoSmithKline Biologicals S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Engerix BDosage form: Suspension, 10 mcg of hepatitis B surface antigen (HBsAg)/0.5 ml; 1 dose (0.5 ml)Active substance: hepatitis B, purified antigenManufacturer: GlaxoSmithKline Biologicals S.A.Prescription requiredDosage form: Suspension, 20 mcg of hepatitis B surface antigen (HBsAg)/ml, 1-dose vaccine for adults; 1 dose (1 ml)Active substance: hepatitis B, purified antigenPrescription requiredDosage form: Suspension, 10 mcg of hepatitis B surface antigen (HBsAg)/0.5 ml single-dose vaccine for children; 1 dose (0.5 ml)Active substance: hepatitis B, purified antigenPrescription required
Alternatives to Engerix B in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Engerix B in Spain
Alternative to Engerix B in Ukraine
Online doctors for Engerix B
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Engerix B – subject to medical assessment and local rules.














