
ENBREL 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PENS


How to use ENBREL 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PENS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Enbrel 50 mg solution for injection in a pre-filled pen
etanercept
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the pack
The information in this leaflet is organized into the following 7 sections:
- What is Enbrel and what is it used for
- What you need to know before you use Enbrel
- How to use Enbrel
- Possible side effects
- Storing Enbrel
- Contents of the pack and other information
- Instructions for use
1. What is Enbrel and what is it used for
Enbrel is a medicine that is made from two human proteins. It blocks the action of another protein in the body that causes inflammation. Enbrel works by reducing inflammation associated with certain diseases.
Enbrel can be used in adults aged 18 years and older to treat moderate or severe rheumatoid arthritis, psoriatic arthritis, severe axial spondyloarthritis, including ankylosing spondylitis, and moderate or severe plaque psoriasis, usually when other treatments have not been effective enough or are not suitable for you.
In the treatment of rheumatoid arthritis, Enbrel is usually used in combination with methotrexate, although it can also be used as a single medicine if treatment with methotrexate is not suitable for you. Enbrel can slow down the damage caused by rheumatoid arthritis to your joints and improve your ability to perform daily activities, whether used alone or in combination with methotrexate.
In patients with psoriatic arthritis with multiple joint involvement, Enbrel can improve your ability to perform normal daily activities. In patients with multiple symmetrical joints that are swollen or painful (e.g., hands, wrists, and feet), Enbrel can delay the progression of structural damage to these joints caused by the disease.
Enbrel is also indicated for the treatment of children and adolescents with the following diseases:
- For the following types of juvenile idiopathic arthritis when treatment with methotrexate has not worked well or is not suitable for them:
- Polyarthritis (with positive or negative rheumatoid factor) and extended oligoarthritis in patients aged 2 years and older.
- Psoriatic arthritis in patients aged 12 years and older.
- For enthesitis-related arthritis in patients aged 12 years and older for whom the use of other commonly used treatments has not worked well or is not suitable for them.
- Severe psoriasis in patients aged 6 years and older who have had an inadequate response to (or are unable to take) phototherapies or other systemic therapies.
2. What you need to know before you use Enbrel
Do not use Enbrel
- if you or the child in your care are allergic to etanercept or any of the other ingredients of Enbrel (listed in section 6). If you or the child experience allergic reactions, such as chest tightness, wheezing, dizziness, or rash, do not inject more Enbrel and contact your doctor immediately.
- if you or the child have or are at risk of developing a severe blood infection called sepsis. If you are not sure, consult your doctor.
- if you or the child have any type of infection. If you are not sure, consult your doctor.
Warnings and precautions
Talk to your doctor before you start using Enbrel.
- Allergic reactions: If you or the child experience allergic reactions such as chest tightness, wheezing, dizziness, or rash, do not inject more Enbrel and contact your doctor immediately.
- Infections/surgery: If you or the child develop a new infection or are about to undergo major surgery, your doctor may want to monitor your treatment with Enbrel.
- Infections/diabetes: Tell your doctor if you or the child have a history of recurrent infections or have diabetes or other disorders that increase the risk of infection.
- Infections/monitoring: Tell your doctor about any recent travel outside of the European region. If you or the child develop symptoms of an infection such as fever, chills, or cough, notify your doctor immediately. Your doctor will decide whether to continue monitoring you or the child for the presence of infections after you or the child stop treatment with Enbrel.
- Tuberculosis: Since cases of tuberculosis have been reported in patients treated with Enbrel, your doctor will examine you for signs and symptoms of tuberculosis before starting Enbrel. This may include a thorough review of your medical history, chest X-ray, and a tuberculosis test. The results of these tests should be recorded on the Patient Information Card. It is very important that you tell your doctor if you or the child have had tuberculosis or have been in close contact with someone who has had tuberculosis. If symptoms of tuberculosis (such as persistent cough, weight loss, lack of energy, mild fever) or any other infection appear during or after treatment, contact your doctor immediately.
- Hepatitis B: Tell your doctor if you or the child have or have had hepatitis B. Your doctor will perform a hepatitis B test before you or the child start treatment with Enbrel. Treatment with Enbrel may reactivate hepatitis B in patients who have been previously infected with the hepatitis B virus. If this happens, you must stop using Enbrel.
- Hepatitis C: Tell your doctor if you or the child have hepatitis C. Your doctor may want to monitor your treatment with Enbrel in case the infection worsens.
- Blood disorders: Tell your doctor immediately if you or the child have signs or symptoms such as persistent fever, sore throat, bruising, bleeding, or paleness. These symptoms may indicate a serious blood problem that requires interruption of treatment with Enbrel.
- Nervous system and vision disorders: Tell your doctor if you or the child have multiple sclerosis, optic neuritis (inflammation of the optic nerves), or transverse myelitis (inflammation of the spinal cord). Your doctor will decide whether Enbrel is a suitable treatment.
- Congestive heart failure: Tell your doctor if you or the child have a history of congestive heart failure, because Enbrel needs to be used with caution in these circumstances.
- Cancer: Tell your doctor if you have or have had lymphoma (a type of blood cancer) or any other cancer before you are given Enbrel.
Patients with severe rheumatoid arthritis who have had the disease for a long time may be at a higher than average risk of developing lymphoma.
Children and adults who are taking Enbrel may have an increased risk of developing lymphoma or other cancers.
Some adolescent and child patients who have received Enbrel or other medicines that work in a similar way to Enbrel have developed cancers, including unusual types, which sometimes resulted in death.
Some patients who receive Enbrel have developed skin cancers. Tell your doctor if you or the child develop any changes in the appearance of the skin or growths on the skin.
- Chickenpox: Tell your doctor if you or the child are exposed to chickenpox while using Enbrel. Your doctor will decide whether preventive treatment for chickenpox is appropriate.
- Latex: The needle cap of the MYCLIC pen is made of latex (dry natural rubber). Contact your doctor before using Enbrel if the needle cap is to be handled by, or if Enbrel is to be administered to, someone with known or possible latex allergy.
- Alcoholism: Enbrel should not be used to treat alcohol-related hepatitis. Please tell your doctor if you or the child in your care have a history of alcoholism.
- Wegener's granulomatosis: Enbrel is not recommended for the treatment of Wegener's granulomatosis, a rare inflammatory disease. If you or the child in your care have Wegener's granulomatosis, discuss this with your doctor.
- Anti-diabetic medicines: Tell your doctor if you or the child have diabetes or are taking medicines to treat diabetes. Your doctor may decide that you or the child need less anti-diabetic medicine while taking Enbrel.
Children and adolescents
Vaccinations: If possible, children should have all their vaccinations up to date before using Enbrel. Some vaccines, such as oral polio vaccine, should not be given while Enbrel is being used. Consult your doctor before you or the child use any vaccine.
Enbrel is not normally used in children under 2 years of age with polyarthritis or extended oligoarthritis, in children under 12 years of age with enthesitis-related arthritis or psoriatic arthritis, or in children under 6 years of age with psoriasis.
Other medicines and Enbrel
Tell your doctor or pharmacist if you or the child are using, have recently used, or might use any other medicines (including anakinra, abatacept, or sulfasalazine), even those not prescribed by your doctor. You or the child must not use Enbrel with medicines that contain the active substances anakinra or abatacept.
Pregnancy and breastfeeding
Enbrel should only be used during pregnancy if clearly necessary. Consult your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant.
If you have received Enbrel during pregnancy, your baby may have a higher risk of getting an infection. Additionally, in one study, more birth defects were seen when the mother had received Enbrel during pregnancy compared to mothers who had not received Enbrel or similar medicines (TNF antagonists), but there was no pattern in the types of birth defects reported. Another study did not find a higher risk of birth defects when the mother had received Enbrel during pregnancy. Your doctor will help you decide whether the benefits of treatment outweigh the potential risk to your baby.
Consult your doctor if you want to breastfeed while being treated with Enbrel. It is important that you inform the pediatrician and other healthcare professionals about the use of Enbrel during pregnancy and breastfeeding before your baby receives any vaccine.
Driving and using machines
It is not expected that the use of Enbrel will affect your ability to drive or use machines.
Enbrel contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to use Enbrel
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are not sure, consult your doctor or pharmacist again.
If you think that the action of Enbrel is too strong or too weak, talk to your doctor or pharmacist.
Your doctor has prescribed a dose of 50 mg of Enbrel. A 25 mg presentation of Enbrel is also available for the administration of 25 mg doses.
Dose for adult patients (aged 18 years and older)
Rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis, including ankylosing spondylitis
The usual dose is 25 mg administered twice a week or 50 mg administered once a week, given as an injection under the skin. However, your doctor may decide on an alternative frequency for injecting Enbrel.
Plaque psoriasis
The usual dose is 25 mg twice a week or 50 mg once a week.
Alternatively, 50 mg may be administered twice a week for up to 12 weeks, followed by 25 mg twice a week or 50 mg once a week.
Your doctor will decide how long you should take Enbrel and whether you need to repeat treatment based on your response. If Enbrel does not have an effect on your disease after 12 weeks, your doctor may tell you to stop using this medicine.
Use in children and adolescents
The suitable dose and frequency of administration will depend on the child's or adolescent's weight and disease. Your doctor will decide the suitable dose for the child and prescribe the most appropriate presentation of Enbrel (10 mg, 25 mg, or 50 mg).
For polyarthritis or extended oligoarthritis in patients aged 2 years and older, or enthesitis-related arthritis or psoriatic arthritis in patients aged 12 years and older, the usual dose is 0.4 mg of Enbrel per kg of body weight (up to a maximum of 25 mg) twice a week, or 0.8 mg of Enbrel per kg of body weight (up to a maximum of 50 mg) once a week.
For psoriasis in patients aged 6 years and older, the usual dose is 0.8 mg of Enbrel per kg of body weight (up to a maximum of 50 mg) once a week. If Enbrel does not have an effect on the child's disease after 12 weeks, your doctor may tell you to stop using this medicine.
Your doctor will give you precise instructions for preparing and calculating the correct dose.
Method and route of administration
Enbrel is given by injection under the skin (subcutaneous injection).
Enbrel can be administered with or without food or drink.
In section 7, "Instructions for use", you will find detailed instructions for injecting Enbrel.The Enbrel solution should not be mixed with any other medicine.
To help you remember, it may be useful to note down the day(s) of the week you should use Enbrel in a diary.
If you use more Enbrel than you should
If you use more Enbrel than you should (either by injecting too much at one time or by using it too often), you should talk to a doctor or pharmacist immediately. Always carry the medicine pack with you, even if it is empty.
If you forget to inject Enbrel
If you miss a dose, you should inject it as soon as you remember, unless the next dose is scheduled for the next day, in which case you should omit the missed dose. Then continue injecting the medicine on the usual day(s). If you do not remember until the day you are due to take the next dose, do not inject a double dose (two doses on the same day) to make up for the missed dose.
If you stop treatment with Enbrel
Your symptoms may return after stopping treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Allergic Reactions
If you observe any of the following reactions, do not inject more Enbrel. Inform your doctor immediately or go to the Emergency Service of the nearest hospital.
- Difficulty swallowing or breathing.
- Swelling of the face, throat, hands, and feet.
- Feeling of nervousness or anxiety, palpitations, sudden reddening of the skin, and/or feeling of heat.
- Severe rash, itching, or urticaria (prominent skin rashes, reddened or pale, often accompanied by itching).
Severe allergic reactions are rare. However, any of the above symptoms may be a sign of an allergic reaction to Enbrel, so you should seek urgent medical attention immediately.
Severe Adverse Effects
If you notice any of the following effects, you or the child may need urgent medical attention.
- Signs of severe infections, such as high fever that may be accompanied by cough, shortness of breath, chills, weakness, or a painful, sensitive, reddened, and warm area of skin or joints.
- Signs of blood disorders, such as bleeding, bruising, or paleness.
- Signs of nervous system disorders, such as numbness or tingling, vision changes, eye pain, or weakness in an arm or leg.
- Signs of heart failure or worsening of heart failure, such as fatigue or shortness of breath with activity, swelling of the ankles, feeling of fullness in the neck or abdomen, shortness of breath at night, or cough, blue discoloration of the nails or around the lips.
- Signs of cancer: cancer can affect any part of the body, including the skin and blood, and the possible signs will depend on the type and location of the cancer. These signs may include weight loss, fever, swelling (with or without pain), persistent cough, presence of lumps or thickening of the skin.
- Signs of autoimmune reactions(in which antibodies that can damage normal body tissues develop) such as pain, itching, weakness, and abnormal breathing, thinking, sensation, or vision.
- Signs of lupus or lupus-like syndromesuch as weight changes, persistent rash, fever, muscle or joint pain, or fatigue.
- Signs of inflammation of blood vesselssuch as pain, fever, reddening or warmth of the skin, or itching.
These adverse effects are rare or infrequent, but they are serious conditions (some of which can be life-threatening). If these signs occur, inform your doctor immediately or go to the Emergency Service of the nearest hospital.
The following are the known adverse effects of Enbrel, grouped by decreasing frequency:
- Very common(may affect more than 1 in 10 people):
Infections (including colds, sinusitis, bronchitis, urinary tract infections, and skin infections); injection site reactions (including bleeding, bruising, reddening, itching, pain, and swelling) (do not occur as frequently after the first month of treatment; some patients have developed an injection site reaction that they had recently used); and headache.
- Common(may affect up to 1 in 10 people):
Allergic reactions; fever; rash; itching; antibodies directed against normal tissues (formation of autoantibodies).
- Uncommon(may affect up to 1 in 100 people):
Severe infections (including pneumonia, non-superficial skin infections, joint infections, blood infections, and generalized infections); worsening of congestive heart failure; low red blood cell count, low white blood cell count, low neutrophil count (a type of white blood cell); low platelet count; skin cancer (excluding melanoma); localized skin swelling (angioedema); urticaria (prominent skin rashes, reddened or pale, often accompanied by itching); eye inflammation, psoriasis (new or worsening); inflammation of blood vessels affecting multiple organs; increased liver enzymes in blood tests (in patients who also receive treatment with methotrexate, the increase in liver enzymes is frequent); abdominal cramps and pain, diarrhea, weight loss, or blood in the stool (signs of intestinal problems).
- Rare(may affect up to 1 in 1,000 people):
Severe allergic reactions (including severe localized skin swelling and wheezing); lymphoma (a type of blood cancer); leukemia (cancer that affects the blood and bone marrow); melanoma (a type of skin cancer); combined low red blood cell count, white blood cell count, and platelet count; nervous system disorders (with severe muscle weakness and symptoms similar to those of multiple sclerosis or inflammation of the optic nerves or spinal cord); tuberculosis; new-onset congestive heart failure; seizures; lupus or lupus-like syndrome (symptoms may include persistent rash, fever, joint pain, and fatigue); skin rash that can lead to severe blistering and peeling of the skin; lichenoid reactions (pruritic reddish-purple skin rash and/or thick white-gray lines on the mucous membranes); autoimmune hepatitis (in patients who also receive treatment with methotrexate, the frequency is uncommon); sarcoidosis (an immune disorder that can affect the lungs, skin, and lymph nodes); lung inflammation or scarring (in patients who also receive treatment with methotrexate, the frequency of lung inflammation or scarring is uncommon); damage to the small filters within the kidneys, leading to impaired kidney function (glomerulonephritis).
- Very rare(may affect up to 1 in 10,000 people):
Failure of the bone marrow to produce crucial blood cells.
- Frequency not known(cannot be estimated from the available data):
Merkel cell carcinoma (a type of skin cancer); Kaposi's sarcoma, a rare cancer related to human herpesvirus 8 infection. Kaposi's sarcoma usually manifests more frequently as purple-colored skin lesions; excessive activation of white blood cells associated with inflammation (macrophage activation syndrome); reactivation of hepatitis B (a liver infection); worsening of a disease called dermatomyositis (inflammation and weakness of the muscles accompanied by skin rash).
Other Adverse Effects in Children and Adolescents
The adverse effects observed in children and adolescents, as well as their frequencies, are similar to those described above.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Enbrel
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and on the pre-filled pen MYCLIC after "EXP". The expiration date is the last day of the month indicated.
Store in a refrigerator (2°C - 8°C). Do not freeze.
Keep the pre-filled pens in the outer packaging to protect them from light.
After removing the pre-filled pen from the refrigerator, wait approximately15 to30 minutes to allow the Enbrel solutioninthe pen to reach room temperature.Do not heat it in any other way. Then, it is recommended to use it immediately.
Enbrel can be stored outside the refrigerator at a maximum temperature of 25°C, and for a single period of up to 4 weeks; after which, the medicine cannot be refrigerated again. Enbrel must be discarded if it has not been used within 4 weeks of removal from the refrigerator. It is recommended that you note the date on which Enbrel was removed from the refrigerator and the date from which Enbrel must be discarded (not later than 4 weeks from the removal of the packaging from the refrigerator).
Inspect the solution in the pen by looking through the transparent inspection window. The solution should be transparent or slightly opalescent, colorless to pale yellow or pale brown, and may contain small white or almost transparent protein particles. This is the normal appearance of Enbrel. Do not use the solution if it is discolored or cloudy, or if it contains particles other than those described above. If you are concerned about the appearance of the solution, contact your pharmacist for assistance.
Medicines should not be thrown away through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Enbrel
The active ingredient of Enbrel is etanercept. Each pre-filled pen MYCLIC of Enbrel contains 50 mg of etanercept.
The other components are sucrose, sodium chloride, L-arginine hydrochloride, sodium phosphate monobasic dihydrate, sodium phosphate dibasic dihydrate, and water for injectable preparations.
Appearance of the Product and Package Contents
Enbrel is presented as an injectable solution in a pre-filled pen (MYCLIC) (injectable solution). The MYCLIC pen contains a transparent injectable solution, colorless to pale yellow or pale brown. Each package contains 2, 4, or 12 pens and 2, 4, or 12 cotton pads with alcohol.
Marketing Authorization Holder:Pfizer Europe MA EEIG Boulevard de la Plaine 17 1050 Brussels Belgium | |
Manufacturer: Pfizer Manufacturing Belgium NV Rijksweg 12, 2870 Puurs-Sint-Amands Belgium |
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Spain
Pfizer, S.L.
Phone: +34 91 490 99 00
Date of the Last Revision of this Leaflet:
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Instructions for Use
Enbrel50mg injectable solution in a pre-filled pen
(etanercept)
For subcutaneous injection only
Introduction
- The following instructions explain how to use the MYCLIC pen to inject Enbrel.
- Read the instructions carefully and follow them step by step.
- Your healthcare professional will show you how to inject Enbrel. Do not attempt to inject yourself until you are sure you understand how to use the MYCLIC pen correctly.
- If you have any questions about how to inject, ask your healthcare professional for help.
Pre-filled MYCLIC Pen
Before Injection
| |||
White needle cap | |||
Gray activation button | Expiration date | Transparent inspection window |
After Injection
| ||
Full inspection window | Open end – safety shield |
Step 1 Prepare for an Enbrel Injection
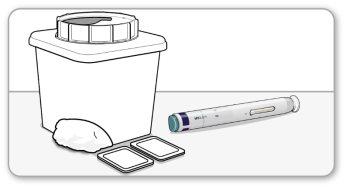
- Gatherthe following materials for each injection on a flat, clean, and well-lit surface:
o A pre-filled MYCLIC pen.
o An alcohol swab.
o A suitable container for sharp objects (not included).
o Cotton balls or clean gauze (not included).
- Do notshake the pen.
- Do notremove the white cap until instructed to do so.
- For a more comfortable injection, leave the pen at room temperature between 15 and 30 minutes with the white cap on.
- Do notheat the pen in any other way.
Step 2 Check the Expiration Date and Dose on the Label
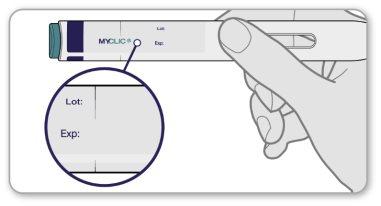
- Checkthe expiration date (month/year) on the pen label.
- Make surethe pen label shows the correct dose.
- If the expiration date has passed or it is not the prescribed dose, do notuse the pen and contact your healthcare professional for help.
Step 3 Inspect the Medication
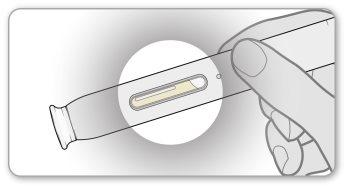
- Inspectthe medication in the pen by looking through the transparent inspection window. The solution should be clear or slightly opalescent, colorless to pale yellow or pale brown, and may contain small white or almost transparent protein particles. This is the normal appearance of Enbrel.
- Do notuse the medication if it has changed color, is cloudy, or has particles other than those described above. If you are concerned about the appearance of the medication, contact your healthcare professional for help.
- Note:you may see an air bubble in the window. This is normal.
Step 4 Choose and Clean the Injection Site
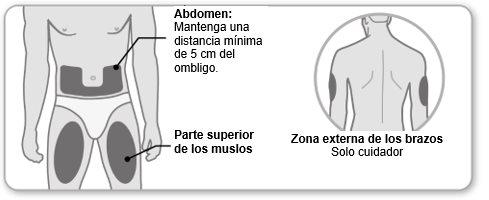
- Choosean injection site in the center of the upper thigh or on the stomach area, 5 cm from the navel. The outer area of the upper arm can also be used by a caregiver.
- Eachinjection should be administered at a minimum distance of 3 cm from the previous injection site. Do notinject into sensitive, bruised, or hardened skin areas. Avoid areas with scars or stretch marks. If you have psoriasis, do notinject directly into any raised, thick, red, or scaly skin patch.
- Cleanthe injection site with soap and water or, if you prefer, with an alcohol swab.
- Letit dry. Do nottouch, fan, or blow on the cleaned injection site.
Step 5 Remove the Needle Cap
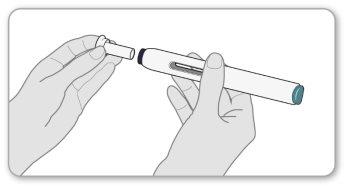
- Removethe white needle cap by pulling it straight off. Do notbend the cap when removing it.
- Do notreplace the cap once it has been removed.
- Once the cap is removed, you will see a purple safety shield that slightly protrudes from the end of the pen. Do notpush the safety shield at the end with your fingers or thumbs.
- Do notuse the pen if it falls with the needle cap removed.
Note:you may notice a drop of liquid on the tip of the needle. This is normal.
Step 6 Press the Pen Firmly Against the Skin
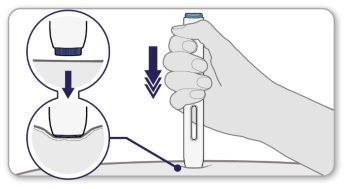
- Pushthe open end of the pen firmly against the skin at a 90-degree angle, so that the purple needle safety shield is completely inside the pen.
Note:you will only be able to press the gray button when the needle shield is fully inserted into the pen.
Pinning or stretching the skin before injection can make the injection site firmer, making it easier to press the injection button.
Step 7 Start the Injection
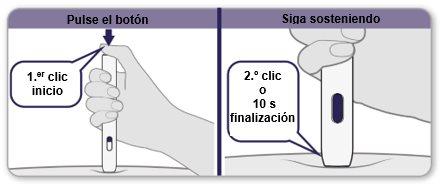
- Pressthe gray button all the way down and you will hear a “click”. The click means the injection has started.
- Continue to holdthe pen firmly against the skin until you hear a second “click”, or until 10 seconds after the first click (whichever comes first).
Note:if you cannot start the injection as described, press the pen more firmly against the skin and then press the gray button again.
Step 8 Remove the Pen from the Skin
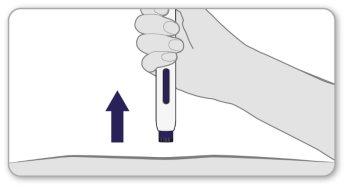
- Removethe pen from the skin by lifting it straight up from the injection site.
- The purple needle safety shield will automatically extend to cover the needle.
Step 9 Check the Inspection Window
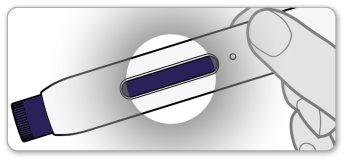
- Checkthe inspection window on the pen. It should be completely purple.
- If the window is not purple, you may not have received a complete dose. Contact your healthcare professional for help. Do notattempt to use the pen again. Do notattempt to use another pen.
- If you notice a blood spot at the injection site, press a cotton ball or gauze over the injection site for 10 seconds. Do notrub the injection site.
Note:the injection button may remain pressed. This is normal.
Step 10 Disposal
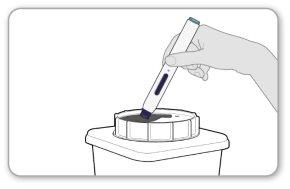
- Disposeof the used pen by following the instructions of your healthcare professional. Do notattempt to recap the pen.
- Do notpress the end of the needle safety shield. If you have any questions, consult your healthcare professional.
—End of instructions—
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ENBREL 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PENSDosage form: INJECTABLE, 25 mgActive substance: etanerceptManufacturer: Samsung Bioepis Nl B.V.Prescription requiredDosage form: INJECTABLE, 50 mgActive substance: etanerceptManufacturer: Samsung Bioepis Nl B.V.Prescription requiredDosage form: INJECTABLE, 50mgActive substance: etanerceptManufacturer: Samsung Bioepis Nl B.V.Prescription required
Online doctors for ENBREL 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PENS
Discuss questions about ENBREL 50 mg SOLUTION FOR INJECTION IN PRE-FILLED PENS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








