
ELTROMBOPAG CIPLA 50 mg FILM-COATED TABLETS

How to use ELTROMBOPAG CIPLA 50 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Eltrombopag Cipla 25 mg film-coated tablets EFG
Eltrombopag Cipla 50 mg film-coated tablets EFG
Eltrombopag Cipla 75 mg film-coated tablets EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Eltrombopag Cipla and what is it used for
- What you need to know before you take Eltrombopag Cipla
- How to take Eltrombopag Cipla
- Possible side effects
- Storage of Eltrombopag Cipla
- Contents of the pack and other information
1. What is Eltrombopag Cipla and what is it used for
Eltrombopag Cipla contains eltrombopag which belongs to a group of medicines called thrombopoietin receptor agonists. It is used to help increase the number of platelets in your blood. Platelets are blood cells that help to prevent or stop bleeding.
- Eltrombopag is used to treat a blood disorder called immune (primary) thrombocytopenia (ITP) in patients aged 1 year and above who have had other treatments (such as corticosteroids or immunoglobulins) that have not worked.
ITP is caused by a low platelet count (thrombocytopenia). People with ITP have a higher risk of bleeding. Symptoms that patients with ITP may notice include petechiae (small, round, flat red spots under the skin), bruising, nosebleeds, bleeding gums, and an inability to stop bleeding if they cut or injure themselves.
- Eltrombopag may also be used to treat low platelet counts (thrombocytopenia) in adults with hepatitis C virus (HCV) infection, if they have had problems with the side effects of treatment with interferon. Many people with hepatitis C have low platelet counts, not only because of the disease but also due to the antiviral treatments used to treat it. Taking eltrombopag may help you complete a full course of antiviral treatment (peginterferon and ribavirin).
- Eltrombopag may also be used in patients with low blood cell counts caused by severe aplastic anemia (SAA). SAA is a disease in which the bone marrow is damaged, leading to a deficiency of red blood cells (anemia), white blood cells (leukopenia), and platelets (thrombocytopenia).
2. What you need to know before you take Eltrombopag Cipla
Do not take Eltrombopag Cipla
if you are allergicto eltrombopag or any of the other ingredients of this medicine (listed in section 6).
Consult your doctorif you think this may apply to you.
Warnings and precautions
Tell your doctor or pharmacist before you start taking this medicine:
- if you have liver problems. People who have a low platelet count and advanced liver disease (such as cirrhosis) have a higher risk of side effects, liver damage that can be fatal, and blood clots. If your doctor thinks that the benefits of taking this medicine outweigh the risks, they will closely monitor you during treatment.
- if you are at risk of having a blood clotin your veins or arteries, or if you have a family history of blood clots.
The risk of having a blood clot may be higherin the following situations:
- if you are older
- if you have been bedridden for a long time
- if you have cancer
- if you are taking the contraceptive pill or hormone replacement therapy
- if you have recently had surgery or have been injured
- if you are very overweight (obese)
- if you smoke
- if you have advanced liver disease
Tell your doctorif you are in any of these situations. Do not take this medicine unless your doctor thinks that the benefits outweigh the risk of having blood clots.
- if you have cataracts(clouding of the lens in the eye).
- if you have another blood disorder, such as myelodysplastic syndrome (MDS). Before you start taking eltrombopag, your doctor will do tests to check that you do not have this disease. If you have MDS and take this medicine, the MDS may get worse.
Tell your doctorif you are in either of these situations.
Eye examinations
Your doctor will recommend that you have an eye examination to check for cataracts. If you do not have regular eye examinations, your doctor will ask you to have one. They will also check your retina (the light-sensitive layer at the back of the eye) for any bleeding in the retina or around it.
You will need to have regular blood tests
Before you start taking eltrombopag, your doctor will do a blood test to check your blood cells, including your platelets. These tests will be repeated regularly while you are taking the medicine.
Blood tests to check your liver
Eltrombopag may cause changes in your liver blood tests that show liver damage - an increase in some liver enzymes, especially bilirubin and alanine/aspartate transaminase. If you are taking interferon-based treatments with eltrombopag to treat low platelet counts due to hepatitis C, some liver problems may get worse.
You will have blood tests before you start taking eltrombopag and regularly while you are taking it to check your liver. You may need to stop taking eltrombopag if the levels of these markers increase too much or if you have any other signs of liver damage.
Read the information on “Liver problems” in section 4 of this leaflet
Blood tests to check your platelet count
If you stop taking eltrombopag, it is likely that your platelet count will fall again within a few days. Your platelet count will be checked and your doctor will tell you what precautions to take.
Very high platelet counts can increase the risk of blood clots. However, blood clots can also occur with normal or low platelet counts. Your doctor will adjust your dose of eltrombopag to make sure that your platelet count does not get too high.
Seek medical help immediatelyif you have any of these signs of a blood clot:
- swelling, painor tenderness in one leg
- sudden difficulty breathing, which may be accompanied by sharp chest pain or rapid breathing
- abdominal pain (stomach), enlarged abdomen, blood in your stools.
Tests to examine your bone marrow
In people with bone marrow problems, medicines like eltrombopag may make these problems worse. Signs of changes in the bone marrow may appear as abnormal blood test results. Your doctor may also do tests to directly check your bone marrow during treatment with eltrombopag.
Checking for bleeding in the gut
If you are taking interferon-based treatments with eltrombopag, you will be monitored for any signs of bleeding in your stomach or intestines after you stop taking this medicine.
Heart monitoring
Your doctor may consider it necessary to monitor your heart while you are taking eltrombopag and may do an electrocardiogram (ECG) test.
Older adults (65 years and over)
There is limited information on the use of eltrombopag in patients aged 65 years and over. Caution should be used when taking eltrombopag if you are 65 years or older.
Children and adolescents
Eltrombopag is not recommended for use in children under 1 year of age with ITP. It is also not recommended for use in children under 18 years of age with low platelet counts due to hepatitis C or severe aplastic anemia.
Other medicines and Eltrombopag Cipla
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines that you buy without a prescription and herbal medicines.
Some common medicines may interact with eltrombopag(including prescription and non-prescription medicines and minerals). These include:
- antacids for indigestion, heartburn, or stomach ulcers(see also section 3 “How to take Eltrombopag Cipla”).
- statins, for lowering cholesterol
- certain medicines for HIV infection, such as lopinavir and/or ritonavir
- ciclosporin, used in transplantsor immune system diseases
- minerals such as iron, calcium, magnesium, aluminum, selenium, and zinc, which may be present in vitamin and mineral supplements(see also section 3 “How to take Eltrombopag Cipla”).
- medicines such as methotrexate and topotecan, used to treat cancer.
Consult your doctorif you are taking any of these medicines. Some should not be taken with eltrombopag, the dose may need to be adjusted, or you may need to change the times when you take them. Your doctor will review the medicines you are taking and recommend alternatives if necessary.
If you are also taking medicines to prevent blood clots, there may be a higher risk of bleeding. Your doctor will discuss this with you.
If you are taking corticosteroids, danazol,and/or azathioprinewith eltrombopag, you may need to reduce the dose or stop treatment with these medicines.
Taking Eltrombopag Cipla with food and drink
Do not take eltrombopag with dairy foods or drinks, as the calcium in these products affects the absorption of the medicine. For more information, see section 3, “How to take Eltrombopag Cipla”.
Pregnancy and breastfeeding
Do not take eltrombopag if you are pregnantunless your doctor recommends it. The effects of eltrombopag during pregnancy are not known.
- Tell your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant.
- Use a reliable method of contraceptionto prevent pregnancy while you are taking eltrombopag.
- If you become pregnant while taking eltrombopag, tell your doctor.
Do not breastfeed while you are taking eltrombopag. It is not known if eltrombopag passes into breast milk.
If you are breastfeedingor plan to breastfeed, tell your doctor.
Driving and using machines
Eltrombopag may cause dizzinessand have other effects that may make you less alert.
Do not drive or use machinesunless you are sure that eltrombopag does not affect you.
Eltrombopag Cipla contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per tablet; this is essentially “sodium-free”.
Eltrombopag Cipla contains orange yellow S (E-110)
It may cause allergic reactions.
3. How to take Eltrombopag Cipla
Take this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
Do not change the dose or stop treatment with eltrombopag unless your doctor tells you to. While you are taking eltrombopag, you will be under the care of a specialist doctor with experience in treating your condition.
How much to take For ITP
Adults and children(aged 6 to 17 years) - the usual starting dose for ITP is one 50 mg tabletof eltrombopag per day. If you are of East or South-East Asian origin, you may need to start with a lower dose of 25 mg.
Children(aged 1 to 5 years) - the usual starting dose for ITP is one 25 mg tabletof eltrombopag per day.
For hepatitis C
Adults- the usual starting dose for hepatitis C is one 25 mg tabletof eltrombopag per day. If you are of East or South-East Asian origin, start with the same dose of 25 mg.
For SAA
Adults- the usual starting dose for SAA is one 50 mg tabletof eltrombopag per day. If you are of East or South-East Asian origin, you may need to start with a lower dose of 25 mg.
Eltrombopag may take 1 to 2 weeks to start working. Depending on your response to eltrombopag, your doctor may recommend that you change your daily dose.
How to take the tablets
Swallow the tablet whole, with water
When to take it
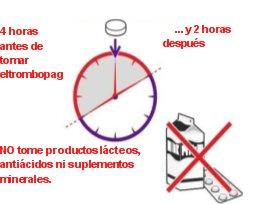
Make sure that -
- in the 4 hours beforeyou take eltrombopag
- and in the 2 hours afteryou take eltrombopag
you do noteat or drink:
- dairy foodssuch as cheese, butter, yogurt, or ice cream
- milk or milkshakes, drinks that contain milk, yogurt, or cream
- antacids, a type of medicine for indigestion and heartburn
- certain vitamin and mineral supplements, including iron, calcium, magnesium, aluminum, selenium, and zinc.
If you do, your body may not absorb the medicine properly.
For more information on what foods and drinks are suitable, ask your doctor.
If you take more Eltrombopag Cipla than you should
Consult your doctor or pharmacist immediately. If possible, show them the pack or this leaflet. You will be monitored for signs or symptoms of side effects and will be given any necessary treatment.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone: 91 562 04 20, stating the medicine and the amount taken.
If you forget to take Eltrombopag Cipla
Take the next dose at the usual time. Do not take more than one dose of eltrombopag per day.
If you stop taking Eltrombopag Cipla
Do not stop taking eltrombopag without first talking to your doctor. If your doctor advises you to stop treatment, your platelet count will be checked every week for 4 weeks. See also “Bleeding or bruising after stopping treatment” in section 4.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Symptoms to which you need to pay attention: consult your doctor
People taking eltrombopag for both ITP and low platelet count associated with hepatitis C may exhibit signs related to potential serious adverse effects. It is essential that you inform your doctor if you develop symptoms.
Increased Risk of Thrombosis
Some people may have an increased risk of suffering a thrombus, and medications like eltrombopag can worsen this problem. The sudden blockage of a blood vessel by a thrombus is a rare adverse effect that can affect up to 1 in 100 people.
 Seek medical help immediately if you experience signs or symptoms of thrombosis, such as:
Seek medical help immediately if you experience signs or symptoms of thrombosis, such as:
- swelling, pain, heat, redness, orsensitivity in one leg.
- sudden difficulty breathing, exceptionally accompanied by acute chest pain or agitated breathing.
- abdominal pain (stomach),enlarged abdomen, blood in your stool.
Liver Problems
Eltrombopag may cause changes that appear in blood tests, which can be signs of liver damage. Liver problems (increased liver enzymes in blood tests) are frequent and can affect up to 1 in 10 people. Other liver problems are rare and can affect up to 1 in 100 people. If you have any signs of liver problems:
- yellowish coloron the skin or in the white area of the eyes (jaundice)
- dark-colored urineunusual.
contact your doctor immediately
Bleeding or Hematoma after Treatment Discontinuation
Within two weeks after discontinuing treatment with eltrombopag, your platelet levels will normally drop to levels similar to those before starting treatment with this medication. A decrease in platelet levels can increase the risk of bleeding or hematoma. Your doctor will check your platelet levels for at least 4 weeks after discontinuing treatment with eltrombopag.
contact your doctor if you experience bleeding or hematoma when stopping eltrombopag.
Some people have bleeding in the digestive systemafter stopping peginterferon, ribavirin, and eltrombopag. Symptoms include:
- black, tar-like stools (stool discoloration is a rare adverse effect that can affect up to 1 in 100 people).
- blood in the stool
- vomiting blood or something that looks like coffee grounds
contact your doctor immediately if you experience any of these symptoms.
The following adverse effects have been reported in relation to treatment with eltrombopag in adult patients with ITP
Very Common Adverse Effects
May affect more than 1 in 10people
- cold
- feeling of dizziness (nausea)
- diarrhea
- cough
- infection of the nose, sinuses, throat, and respiratory tract (upper respiratory tract infection)
- back pain
Very Common Adverse Effects that may appear in Blood Tests:
- increase in liver enzymes (alanine aminotransferase (ALT))
Common Adverse Effects
May affect up to 1 in 10people
- muscle pain, muscle spasm, muscle weakness
- bone pain
- heavy menstruation
- throat irritation and swallowing difficulties
- eye problems including abnormal eye tests, dry eye, eye pain, and blurred vision
- vomiting
- flu
- cold sores
- pneumonia
- breast tenderness and swelling (gynecomastia)
- tonsillitis
- lung infection, sinusitis, and throat infection
- gum inflammation
- loss of appetite
- tingling, itching, or numbness sensation
- decreased skin sensitivity
- drowsiness
- ear pain
- pain, swelling, and sensitivity in one leg (usually the calf) with hot skin in the affected area (signs of a blood clot in a deep vein)
- localized swelling filled with blood from a ruptured blood vessel (hematoma)
- hot flashes
- mouth alterations including dryness or irritation in the mouth, tongue sensitivity, gum bleeding, mouth ulcers
- runny nose
- toothache
- abdominal pain
- abnormal liver function
- skin changes including excessive sweating, itchy rash, red spots, skin appearance changes
- hair loss
- foamy or bubbly urine (signs of protein in the urine)
- high temperature, feeling of heat
- chest pain
- feeling of weakness
- sleeping problems, depression
- migraine
- decreased vision
- feeling of dizziness (vertigo)
- gas
Common Adverse Effects that may appear in Blood Tests:
- decrease in red blood cell count (anemia)
- decrease in platelet count (thrombocytopenia)
- decrease in white blood cell count
- decrease in hemoglobin levels
- increase in eosinophil count
- increase in white blood cell count (leukocytosis)
- increase in uric acid level
- decrease in potassium levels
- increase in creatinine levels
- increase in alkaline phosphatase levels
- increase in liver enzymes (aspartate aminotransferase (AST))
- increase in blood bilirubin (a substance produced by the liver)
- increase in some protein levels
Rare Adverse Effects
May affect up to 1 in 100people:
- allergic reaction
- disruption of blood supply to parts of the heart
- sudden difficulty breathing, especially when accompanied by acute chest pain and/or agitated breathing, which could be signs of a pulmonary thrombus (see “Increased Risk of Thrombosis” above in section 4)
- partial loss of lung function caused by a blockage in the pulmonary artery
- possible pain, swelling, and/or redness around a vein that could be signs of thrombosis in a vein
- yellowish skin and/or abdominal pain that could be signs of a bile duct obstruction, liver injury, liver damage due to inflammation (see “Liver Problems” above in section 4)
- liver damage due to medication
- rapid or irregular heartbeat, discoloration of the skin, cardiac rhythm alterations (prolonged QT interval) that could be a sign of a heart and blood vessel disorder.
- blood clots
- hot flashes
- joint pain and swelling due to uric acid (gout)
- lack of interest, mood changes, uncontrolled crying
- balance problems, speech and nervous system alterations, tremors
- pain or abnormal sensations in the skin
- paralysis of one side of the body
- migraine with aura
- nerve pain
- dilation or swelling of blood vessels that cause headaches
- eye problems, including increased tearing, blurred vision, retinal hemorrhage, dry eye
- nose, throat, and sinus problems, breathing difficulties while sleeping
- blisters/pain in mouth and throat
- loss of appetite
- digestive system problems, including frequent bowel movements, food poisoning, blood in stool, vomiting blood
- rectal bleeding, changes in stool color, abdominal swelling, constipation
- mouth alterations, including dryness or irritation in the mouth, tongue pain, gum bleeding, mouth ulcers
- sunburn
- feeling of heat, anxiety sensation
- redness or swelling around wounds
- bleeding around a catheter (if you have one) in the skin
- feeling of a foreign body
- kidney problems, including kidney inflammation, frequent urination at night, kidney failure, white blood cells in urine
- cold sweat
- feeling of general discomfort
- skin infection
- skin changes, including skin discoloration, peeling, redness, itching, and sweating
- muscle weakness
- colon and rectal cancer
Rare Adverse Effects that may appear in Blood Tests:
- changes in the shape of white blood cells
- presence of immature white blood cells that may indicate certain diseases
- increase in platelet count
- decrease in calcium levels
- decrease in red blood cell count (anemia) caused by excessive destruction of red blood cells (hemolytic anemia)
- increase in myelocytes
- increase in neutrophils
- increase in blood urea
- increase in protein in urine
- increase in albumin levels in blood
- increase in total protein levels
- decrease in albumin levels in blood
- increase in urine pH
- increase in hemoglobin levels
The following adverse effects have been reported in relation to treatment with eltrombopag in children (1 to 17 years) with ITP
If these adverse effects worsen, please inform your doctor, pharmacist, or nurse.
Very Common Adverse Effects
May affect more than 1 in 10children
- infection of the nose, sinuses, throat, and upper respiratory tract, cold (upper respiratory tract infection)
- diarrhea
- abdominal pain
- cough
- high temperature
- feeling of dizziness (nausea)
Common Adverse Effects
May affect up to 1 in 10children
- difficulty sleeping (insomnia)
- toothache
- throat and nose pain
- itching, runny nose, or stuffiness
- throat irritation, runny nose, nasal congestion, and sneezing
- mouth alterations, including dryness, irritation in the mouth, tongue sensitivity, gum bleeding, mouth ulcers
The following adverse effects have been reported in relation to treatment with eltrombopag in combination with peginterferon and ribavirin in patients with HCV
Very Common Adverse Effects
May affect more than 1 in 10people:
- headache
- loss of appetite
- cough
- feeling of dizziness (nausea), diarrhea
- muscle pain, muscle weakness
- itching
- feeling of fatigue
- fever
- hair loss
- feeling of weakness
- flu-like discomfort
- swelling of hands or feet
- chills
Very Common Adverse Effects that may appear in Blood Tests:
- decrease in red blood cell count (anemia)
Common Adverse Effects
May affect up to 1 in 10people:
- urinary tract infection
- inflammation of the nasal passages, throat, and mouth, flu-like symptoms, dryness, irritation, or inflammation of the mouth, toothache
- infection of the nose, sinuses, throat, and respiratory tract, cold (upper respiratory tract infection), bronchial inflammation
- weight loss
- sleep disorders, abnormal drowsiness, depression, anxiety
- dizziness, attention and memory problems, mood changes
- decreased brain function due to liver damage
- tingling or numbness in hands and feet
- fever, headache
- eye problems, including cataracts, dry eye, small yellow deposits in the retina, yellowish color in the white area of the eyes
- retinal bleeding
- feeling of dizziness (vertigo)
- rapid or irregular heartbeat (palpitations), breathing difficulties
- cough with phlegm, runny nose, flu (influenza), cold sores, throat irritation, and swallowing difficulties
- digestive system alterations, including vomiting, stomach pain, indigestion, constipation, stomach swelling, taste alterations, hemorrhoids, abdominal pain/discomfort, stomach swelling
- toothache
- liver problems, including liver tumor, yellowish color in the white area of the eyes or skin (jaundice), liver damage due to medication (see “Liver Problems” above in section 4)
- skin changes, including rash, dry skin, eczema, skin redness, itching, excessive sweating, unusual skin growth, hair loss
- joint pain, back pain, bone pain, pain in the limbs (arms, legs, hands, and feet), muscle spasms
- irritability, feeling of general discomfort, skin reactions such as redness or swelling and pain at the injection site, chest pain, and discomfort, fluid retention in the body or limbs that causes swelling
- depression, anxiety, sleep problems, nervousness
Common Adverse Effects that may appear in Blood Tests:
- increase in blood sugar (glucose) levels
- decrease in white blood cell count
- decrease in neutrophil count
- decrease in blood albumin levels
- decrease in hemoglobin levels
- increase in blood bilirubin levels (a substance produced by the liver)
- changes in the enzymes that control blood coagulation
Rare Adverse Effects
May affect up to 1 in 100people:
- pain when urinating
- heart rhythm alterations (prolonged QT interval)
- stomach flu (gastroenteritis), throat pain
- blisters/pain in the mouth, stomach inflammation
- skin changes, including skin color changes, peeling, skin redness, itching, and nocturnal sweating
- blood clots in the liver veins (possible liver damage and/or digestive system damage)
- poor blood clotting in small blood vessels with kidney failure
- itching and bruising at the injection site, chest discomfort
- decrease in red blood cell count (anemia) caused by massive destruction of red blood cells (hemolytic anemia)
- confusion, agitation
- liver failure
The following adverse effects have been observed in relation to treatment with eltrombopag in patients with severe aplastic anemia (SAA):
If these adverse effects worsen, please inform your doctor, pharmacist, or nurse.
Very Common Adverse Effects
May affect more than 1 in 10people:
- cough
- headache
- pain in the mouth and throat
- diarrhea
- dizziness, nausea
- joint pain (arthralgia)
- pain in the limbs (arms, legs, hands, and feet)
- dizziness (vertigo)
- feeling very tired
- fever
- chills
- eye itching
- blisters in the mouth
- gum bleeding
- abdominal pain
- muscle spasms
Very Common Adverse Effects that may appear in a Blood Test
- abnormal changes in bone marrow cells
- increase in liver enzymes (aspartate aminotransferase (AST))
Common Adverse Effects
May affect up to 1 in 10people:
- anxiety
- depression
- feeling cold
- feeling of general discomfort
- eye problems, including vision problems, cataracts, eye floaters, dry eye, eye itching, yellowish color in the white area of the eyes or skin
- nosebleeds
- digestive problems, including difficulty swallowing, mouth pain, tongue swelling, vomiting, loss of appetite, stomach pain/discomfort, stomach swelling
flatulence/gas, constipation, alterations in intestinal motility that can cause constipation, bloating, diarrhea, and/or the aforementioned symptoms, changes in stool color
- fainting
- skin problems, including red or purple spots due to bleeding under the skin (petechiae), rash, itching, hives, skin lesions
- back pain
- muscle pain
- bone pain
- weakness (asthenia)
- swelling of the lower limbs due to fluid accumulation
- abnormal urine color
- disruption of blood flow to the spleen (splenic infarction)
- runny nose
Common Adverse Effects that may appear in a Blood Test
- increase in some enzymes due to muscle breakdown (creatine phosphokinase)
- iron accumulation in the body (iron overload)
- decrease in blood sugar levels (hypoglycemia)
- increase in blood bilirubin levels (a substance produced by the liver)
- decrease in white blood cell count
Adverse Effects of Unknown Frequency
Frequency cannot be estimated from the available data
- skin discoloration
- skin darkening
- liver damage due to medication
Reporting of Adverse Effects
If you experience any type of adverse effect, contact your doctor, pharmacist, or nurse.
5. Storage of Eltrombopag Cipla
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging and on the blister after "CAD". The expiry date is the last day of the month indicated.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE Point of the pharmacy. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Eltrombopag Cipla film-coated tablets EFG
The active substance is eltrombopag.
25 mg film-coated tablets
Each film-coated tablet contains eltrombopag olamine equivalent to 25 mg of eltrombopag.
50 mg film-coated tablets
Each film-coated tablet contains eltrombopag olamine equivalent to 50 mg of eltrombopag.
75 mg film-coated tablets
Each film-coated tablet contains eltrombopag olamine equivalent to 75 mg of eltrombopag.
The other components (excipients) are:
Core of the tablet: maltose, microcrystalline cellulose, sodium carboxymethyl starch (type A), povidone K30, and magnesium stearate.
Tablet coating:
25 mg film-coated tablets: poly (vinyl alcohol), talc, glycerol caprylocaprate and dicaprylocaprate, sodium lauryl sulfate, titanium dioxide (E-171), and indigo carmine (E-132).
50 mg film-coated tablets: poly (vinyl alcohol), talc, glycerol caprylocaprate and dicaprylocaprate, sodium lauryl sulfate, titanium dioxide (E-171), and orange yellow S (E-110).
75 mg film-coated tablets: poly (vinyl alcohol), talc, glycerol caprylocaprate and dicaprylocaprate, sodium lauryl sulfate, titanium dioxide (E-171), and red iron oxide (E-172).
Appearance and package contents of the product
Eltrombopag Cipla 25 mg film-coated tablets EFG are blue, round, biconvex (approximately 7.1 mm in diameter) with "E2" engraved on one face.
Eltrombopag Cipla 50 mg film-coated tablets EFG are orange, round, biconvex (approximately 8.2 mm in diameter) with "E5" engraved on one face.
Eltrombopag Cipla 75 mg film-coated tablets EFG are dark pink, round, biconvex (approximately 10.1 mm in diameter) with "E7" engraved on one face.
They are supplied in OPA/Al/PVC/Al blisters in a carton containing 14, 28, or 84 film-coated tablets.
Only some pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Cipla Europe NV
De Keyserlei 58-60, Box 19,
2018, Antwerp
Belgium.
Manufacturer
Bluepharma - Indústria Farmacêutica, S.A.
São Martinho do Bispo
3045-016 Coimbra
Portugal
You can request more information about this medicine by contacting the local representative of the marketing authorization holder
Cipla Europe NV branch in Spain,
C/Guzmán el Bueno, 133 Edificio Britannia,
28003, Madrid
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany: Eltrombopag Cipla Filmtabletten
Spain: Eltrombopag Cipla film-coated tablets EFG
Italy: Eltrombopag Cipla compresse rivestite con film
Portugal: Eltrombopag Cipla comprimidos revestidos por película
Date of the last revision of this leaflet: January 2025
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ELTROMBOPAG CIPLA 50 mg FILM-COATED TABLETSDosage form: TABLET, 12.5 mgActive substance: eltrombopagManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 25 mgActive substance: eltrombopagManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 50 mgActive substance: eltrombopagManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for ELTROMBOPAG CIPLA 50 mg FILM-COATED TABLETS
Discuss questions about ELTROMBOPAG CIPLA 50 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















