Elocta 3000 IU powder and solvent for injectable solution

How to use Elocta 3000 IU powder and solvent for injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ELOCTA 250 UI Powder and Solvent for Solution for Injection
ELOCTA 500 UI Powder and Solvent for Solution for Injection
ELOCTA 750 UI Powder and Solvent for Solution for Injection
ELOCTA 1000 UI Powder and Solvent for Solution for Injection
ELOCTA 1500 UI Powder and Solvent for Solution for Injection
ELOCTA 2000 UI Powder and Solvent for Solution for Injection
ELOCTA 3000 UI Powder and Solvent for Solution for Injection
ELOCTA 4000 UI Powder and Solvent for Solution for Injection
efmoroctocog alfa (recombinant coagulation factor VIII)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is ELOCTA and what is it used for
- What you need to know before you use ELOCTA
- How to use ELOCTA
- Possible side effects
- Storage of ELOCTA
- Contents of the pack and other information
1. What is ELOCTA and what is it used for
ELOCTA contains the active substance efmoroctocog alfa, a recombinant coagulation factor VIII, Fc fusion protein. Factor VIII is a protein that is naturally produced by the body and is necessary for blood to clot and stop bleeding. ELOCTA is a medicine used to treat and prevent bleeding in patients of all ages with hemophilia A (a bleeding disorder caused by a lack of factor VIII).
ELOCTA is prepared using recombinant technology without the addition of any human or animal component in the manufacturing process.
How ELOCTA works
In patients with hemophilia A, factor VIII is missing or does not work properly. ELOCTA is used to replace the missing or deficient factor VIII. ELOCTA increases factor VIII levels in the blood and temporarily corrects the tendency to bleed.
2. What you need to know before you use ELOCTA
Do not use ELOCTA:
- if you are allergic to efmoroctocog alfa or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using ELOCTA.
- There is a small chance that you may have an allergic reaction (anaphylactic reaction) to ELOCTA. Signs of allergic reactions include itching all over the body, hives, feeling of tightness in the chest, difficulty breathing, and low blood pressure. If any of these symptoms occur, stop the injection immediately and contact your doctor.
- The formation of inhibitors (antibodies) is a known complication that can occur during treatment with all factor VIII medicines. These inhibitors, especially in large quantities, can prevent the treatment from working properly, so you and your child will be closely monitored for the development of such inhibitors. If your bleeding or your child's bleeding is not being controlled with ELOCTA, contact your doctor immediately.
Cardiovascular events
If you have heart disease or are at risk of heart disease, be careful when using factor VIII medicines and talk to your doctor.
Catheter-related complications
If you need a central venous access device (CVAD), consider the risk of CVAD-related complications, including local infections, bacteria in the blood, and thrombosis at the catheter insertion site.
Documentation
We strongly recommend that each time ELOCTA is administered, the product name and batch number are recorded.
Other medicines and ELOCTA
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
No effects on the ability to drive or use machines have been observed.
ELOCTA contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial; this is essentially "sodium-free".
However, depending on your body weight and dose, you may receive more than one vial, which should be taken into account if you are on a low-sodium diet.
3. How to use ELOCTA
Treatment with ELOCTA will be started by a doctor who is experienced in the care of patients with hemophilia. Follow exactly the administration instructions for this medicine given by your doctor (see section Instructions for preparation and administration). If you are unsure, talk to your doctor, pharmacist, or nurse.
ELOCTA is given by injection into a vein. Your doctor will calculate your dose of ELOCTA (in International Units or "IU") based on your individual needs for factor VIII replacement treatment and whether it is used for prevention or treatment of bleeding. Talk to your doctor if you think you are not getting control of your bleeding with the dose you are receiving.
How often you need an injection will depend on how well ELOCTA is working for you. Your doctor will do the necessary laboratory tests to make sure you have adequate factor VIII levels in your blood.
Treatment of bleeding
The dose of ELOCTA is calculated based on your body weight and the desired factor VIII levels. The target factor VIII levels depend on the severity and location of the bleeding.
Prevention of bleeding
The usual dose of ELOCTA is 50 IU per kilogram of body weight, given every 3 to 5 days. Your doctor may adjust the dose within the range of 25 to 65 IU per kilogram of body weight. In some cases, especially in younger patients, shorter dosing intervals or higher doses may be needed.
Use in children and adolescents
ELOCTA can be used in children and adolescents of all ages. In children under 12 years of age, higher doses or more frequent injections may be needed.
If you use more ELOCTA than you should
Tell your doctor as soon as possible. Follow exactly the administration instructions for ELOCTA given by your doctor. If you are unsure, talk to your doctor, pharmacist, or nurse.
If you forget to use ELOCTA
Do not take a double dose to make up for forgotten doses. Take your dose as soon as you remember and then continue with your normal dosing schedule. If you are not sure what to do, talk to your doctor or pharmacist.
If you stop using ELOCTA
Do not stop using ELOCTA without talking to your doctor. If you stop using ELOCTA, you may no longer be protected against bleeding or an existing bleed may not stop.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If a severe and sudden allergic reaction (anaphylactic reaction) occurs, the injection should be stopped immediately. Contact your doctor immediately if you experience any of the following symptoms of allergic reactions: swelling of the face, rash, itching all over the body, hives, feeling of tightness in the chest, difficulty breathing, and low blood pressure.
In previously untreated children with factor VIII medicines (see section 2) inhibitors may occur very frequently (more than 1 in 10 patients); however, in patients who have received prior treatment with factor VIII (more than 150 days of treatment), the risk is infrequent (less than 1 in 100 patients). If this happens, the medicines may stop working properly and you may experience persistent bleeding. In this case, contact your doctor immediately.
The following side effects may occur with this medicine.
Uncommon side effects (may affect up to 1 in 100 people)
Headache, dizziness, taste disturbances, slow heart rate, high blood pressure, flushing, pain at the injection site, cough, lower abdominal pain, skin rash, papular rash, catheter-related thrombosis, joint swelling, muscle pain, back pain, joint pain, general malaise, chest pain, feeling of cold, feeling of heat, and low blood pressure.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of ELOCTA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vial after "EXP". The expiry date refers to the last day of the month shown.
Do not use this medicine if it has been stored at room temperature for more than 6 months.
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Store in the original package to protect from light.
Alternatively, ELOCTA can be stored at room temperature (up to 30°C) for a single period not exceeding 6 months. Record the date when ELOCTA is removed from the refrigerator and stored at room temperature on the carton. After storage at room temperature, the medicine should not be returned to the refrigerator.
Once ELOCTA has been prepared, it should be used immediately. If it is not possible to use the prepared ELOCTA solution immediately, it should be used within 6 hours. Do not refrigerate the prepared solution. Protect the prepared solution from direct sunlight.
The prepared solution should be clear to slightly opalescent and colorless. Do not use this medicine if you notice it is cloudy or contains visible particles.
Dispose of any unused solution properly. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
ELOCTA Composition
- The active ingredient is efmoroctocog alfa (recombinant coagulation factor VIII, Fc fusion protein). Each vial of ELOCTA nominally contains 250, 500, 750, 1,000, 1,500, 2,000, 3,000, or 4,000 IU of efmoroctocog alfa.
- The other components are sucrose, sodium chloride, L-histidine, calcium chloride dihydrate, polysorbate 20, sodium hydroxide, hydrochloric acid, and water for injectable preparations. See section 2 if you are on a low-sodium diet.
Product Appearance and Container Contents
ELOCTA is presented as a powder and solvent for solution for injection. The powder is a white to off-white powder or cake. The solvent supplied for the preparation of the solution for injection is a clear and colorless solution. After preparation, the solution for injection is clear to slightly opalescent and colorless.
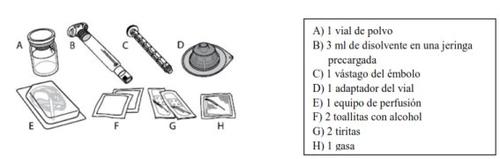
Each ELOCTA container contains 1 vial of powder, 3 mL of solvent in a prefilled syringe, 1 plunger rod, 1 vial adapter, 1 infusion set, 2 alcohol swabs, 2 band-aids, and 1 gauze.
Marketing Authorization Holder and Manufacturer
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm,
Sweden
Date of Last Revision of this Leaflet: 01/2021
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Turn the leaflet over to read the preparation and administration instructions.
Preparation and Administration Instructions
ELOCTA is administered by intravenous (IV) injection after dissolving the injectable powder with the solvent supplied in the prefilled syringe. The ELOCTA container contains:
ELOCTA must not be mixed with other injectable solutions or infusion solutions.
Wash your hands before opening the container.
Preparation:
|
|
|
|
|
|
|
|
|
|
|
Do not shake. |
|
|
|
Note: If using more than one vial of ELOCTA per injection, each vial must be prepared separately according to the previous instructions (steps 1 to 13) and the solvent syringe must be removed, leaving the vial adapter in place. A single larger luer lock syringe may be used to withdraw the prepared contents from each of the vials. |
Note: If the solution is not to be used immediately, the syringe cap should be carefully replaced over the syringe tip. Do not touch the syringe tip or the inside of the cap. After preparation, ELOCTA can be stored at room temperature for a maximum of 6 hours before administration. After this time, the prepared ELOCTA solution must be discarded. Protect it from direct sunlight. |
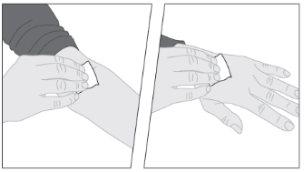
Administration (Intravenous Injection):
ELOCTA should be administered using the infusion set (E) provided in the container.
|
|
|
|
|
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Elocta 3000 IU powder and solvent for injectable solutionDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for Elocta 3000 IU powder and solvent for injectable solution
Discuss questions about Elocta 3000 IU powder and solvent for injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





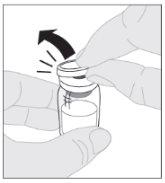 Place the vial on a clean and flat surface. Remove the plastic closure cap from the ELOCTA vial.
Place the vial on a clean and flat surface. Remove the plastic closure cap from the ELOCTA vial.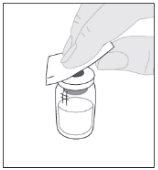 Clean the top of the vial with one of the alcohol swabs (F) provided in the container and let it air dry. Do not touch the top of the vial or allow it to come into contact with anything once cleaned.
Clean the top of the vial with one of the alcohol swabs (F) provided in the container and let it air dry. Do not touch the top of the vial or allow it to come into contact with anything once cleaned.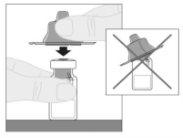
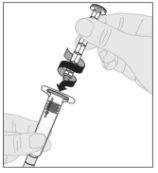 Attach the plunger rod (C) to the solvent syringe by inserting the tip of the plunger rod into the syringe's plunger opening. Turn the plunger rod clockwise until it is securely seated on the syringe plunger.
Attach the plunger rod (C) to the solvent syringe by inserting the tip of the plunger rod into the syringe's plunger opening. Turn the plunger rod clockwise until it is securely seated on the syringe plunger.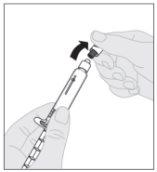 Remove the tamper-evident plastic cap from the solvent syringe by bending it at the perforation until it breaks. Set the cap aside with the top facing down on a flat surface. Do not touch the inside of the cap or the syringe tip.
Remove the tamper-evident plastic cap from the solvent syringe by bending it at the perforation until it breaks. Set the cap aside with the top facing down on a flat surface. Do not touch the inside of the cap or the syringe tip.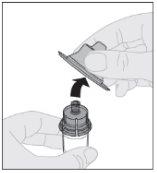
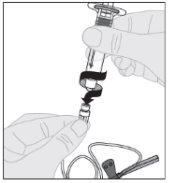
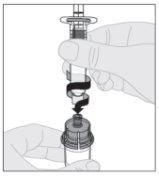 Connect the solvent syringe to the vial adapter by inserting the syringe tip into the adapter opening. Push firmly and turn the syringe clockwise until it is securely connected.
Connect the solvent syringe to the vial adapter by inserting the syringe tip into the adapter opening. Push firmly and turn the syringe clockwise until it is securely connected.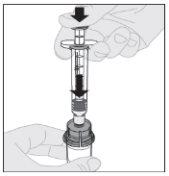 Slowly press the plunger rod downward to inject all of the solvent into the ELOCTA vial.
Slowly press the plunger rod downward to inject all of the solvent into the ELOCTA vial.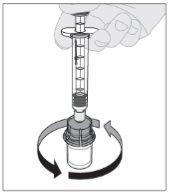
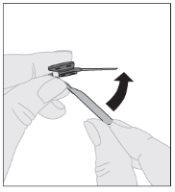
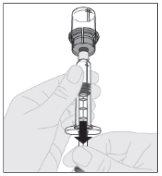 Ensuring the syringe plunger rod is still fully pressed downward, invert the vial. Slowly pull the plunger rod downward to transfer all of the solution into the syringe through the vial adapter.
Ensuring the syringe plunger rod is still fully pressed downward, invert the vial. Slowly pull the plunger rod downward to transfer all of the solution into the syringe through the vial adapter.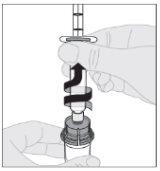 Disconnect the syringe from the vial adapter by gently pulling the vial while turning it counterclockwise.
Disconnect the syringe from the vial adapter by gently pulling the vial while turning it counterclockwise.