ELADYNOS 80 micrograms/dose Injectable solution in pre-filled pen

How to use ELADYNOS 80 micrograms/dose Injectable solution in pre-filled pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Eladynos 80 micrograms/dose solution for injection in pre-filled pen
abaloparatide
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Eladynos and what is it used for
- What you need to know before you use Eladynos
- How to use Eladynos
- Possible side effects
- Storage of Eladynos
- Contents of the pack and further information
1. What is Eladynos and what is it used for
Eladynos contains the active substance abaloparatide. It is used to treat osteoporosis in women after menopause.
Osteoporosis is especially common in women after menopause. This disease causes thinning and fragility of the bones. If you have osteoporosis, you have a higher risk of bone fractures, especially in the spine, hips, and wrists.
This medicine is used to strengthen bones and reduce the risk of bone fractures.
2. What you need to know before you use Eladynos
Do not use Eladynos if
- you are allergic to abaloparatide or any of the other ingredients of this medicine (listed in section 6)
- you are pregnant or breastfeeding
- you may become pregnant
- you have high levels of calcium in the blood
- your kidney function is severely reduced
- you have high levels of the enzyme alkaline phosphatase in the blood without any apparent cause
- you have received radiation therapy to the bones
- you have been diagnosed with bone cancer or other cancers that have spread to the bones
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Eladynos or during treatment if:
- you feel dizzy when standing up, which may be due to a drop in blood pressure. Some patients may feel dizzy or have a rapid heartbeat in the 4 hours following the injection of Eladynos. The first injection(s) should be performed under the guidance of a healthcare professional, who may keep you under observation for the first hour after the injection. Always inject Eladynos in a place where you can sit or lie down if you feel dizzy.
- you have heart disease or high blood pressure. Your doctor may want to monitor you more closely.
- you have nausea, vomiting, constipation, low energy, or muscle weakness on a continuous basis. These may be signs of high levels of calcium in the blood.
- you have high levels of calcium in the urine or have had kidney stones. Eladynos may worsen your condition.
Before starting treatment with Eladynos, you will undergo blood pressure and heart checks.
The recommended treatment duration with Eladynos should not exceed 18 months.
Children and adolescents
Abaloparatide should not be used in children and adolescents under 18 years of age.
Other medicines and Eladynos
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, tell your doctor or pharmacist if you are taking:
- digoxin or digitalis: medicines used to treat heart weakness, as calcium levels in the blood may increase with the use of abaloparatide;
- medicines to control high blood pressure, as they may worsen symptoms of low blood pressure, such as dizziness.
Pregnancy and breastfeeding
Do not use Eladynos if you are pregnant, may become pregnant, or are breastfeeding.
Driving and using machines
Some patients may feel dizzy after the injection of this medicine. If you feel dizzy, do not drive or use machines until you feel better.
Eladynos contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially 'sodium-free'.
3. How to use Eladynos
Follow the instructions for administration of this medicine exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist again.
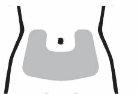
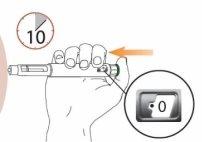
The recommended dose is one injection (80 µg) once a dayunder the skin in the lower abdomen (belly). See the shaded area in gray in the first figure in step 5 of the 'Instructions for use' section at the end of the leaflet.
Preferably, inject Eladynos at the same time every day to help you remember to use the medicine.
Do not inject Eladynos in the same place on the abdomen on consecutive days. Change the injection site every day to reduce the risk of injection site reactions. Administer the injection only in normal skin. Do not administer the injection in areas where the skin is painful to the touch, has a bruise, or is red, scaly, or hardened. Avoid areas with scars or stretch marks.
Follow the 'Instructions for use'carefully at the end of the leaflet.
Your doctor may advise you to take calcium and vitamin D supplements. Your doctor will tell you how much to take per day.
Duration of use
Inject Eladynos daily for the period prescribed by your doctor. The maximum total treatment duration with Eladynos should not exceed 18 months.
If you use more Eladynos than you should
If you accidentally use more Eladynos than you should, inform your doctor or pharmacist. The effects that can be expected from an overdose are, among others, nausea, vomiting, dizziness (especially when standing up quickly), rapid heartbeat, and headache.
If you forget to use Eladynos
If you forget a dose, administer it as soon as possible within 12 hours of the scheduled time. Then, inject the next dose the following day at the usual time.
If more than 12 hours have passed since the time you should have used Eladynos, omit the missed dose. Then, inject the next dose the following day as usual.
Do not use a double dose to make up for missed doses. Do not use more than one injection on the same day.
If you stop treatment with Eladynos
Consult your doctor before stopping treatment. Your doctor will advise you and decide how long you should receive treatment with Eladynos.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop treatment with Eladynos and contact your doctor immediately or go to the emergency department if you experience the following symptoms (severe allergic reaction):
Swelling of the face or tongue, wheezing, and difficulty breathing, hives, itching, and red skin, severe vomiting or diarrhea, and dizziness or loss of consciousness (frequency not known). These symptoms can be severe and potentially life-threatening.
Other side effects may occur with the following frequencies:
Very common(may affect more than 1 in 10 people)
- increased calcium levels in urine tests
- dizziness; see section 2 'Warnings and precautions'
Common(may affect up to 1 in 10 people)
- increased calcium levels in blood tests
- increased uric acid levels in blood tests
- headache
- palpitations, rapid heartbeat
- increased blood pressure
- abdominal pain
- constipation, diarrhea
- nausea, vomiting
- weakness, fatigue, malaise
- injection site reactions such as bruising, rash, and pain
- pain in the bones, joints, hands, feet, or back
- muscle spasms (in the back and legs)
- difficulty sleeping
- kidney stones
- itching, rash
Uncommon(may affect up to 1 in 100 people)
- allergic reactions
- feeling of abdominal distension
- pain
- feeling of dizziness or lightheadedness when standing up or sitting down due to a drop in blood pressure
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Eladynos
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the pen after EXP. The expiry date is the last day of the month shown.
Before first use, store in a refrigerator (between 2°C and 8°C). Do not freeze. Avoid placing the pens near the freezer compartment of the refrigerator to prevent freezing. Do not use Eladynos if it has been frozen.
After first use, store below 25°C and discard after 30 days.
Eladynos should only be stored at room temperature (below 25°C) for 30 days. Write the date of day 1 in the space provided on the carton. Day 1 is the date of the first use or the date the pen was removed from the refrigerator. The purpose is to ensure that you do not use the pen for more than 30 consecutive days or store the pen for more than 30 days. After 30 days, dispose of the pen according to local requirements, even if it still contains unused medicine.
- Dispose of the used pen before opening a new Eladynos pen to avoid possible confusion.
- Do not store the pen with the needle attached.
- Do not use this medicine if the solution is cloudy, has color, or contains visible particles.
Medicines and needles should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines and needles no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Eladynos Composition
- The active ingredient is abaloparatide.
Each 40 microliter dose contains 80 micrograms of abaloparatide.
Each pre-filled pen contains 3 mg of abaloparatide in 1.5 ml of solution (which is equivalent to 2 mg per milliliter).
- The other components are:
- phenol
- water for injectable preparations
- sodium acetate trihydrate (see section 2 "Eladynos contains sodium") and acetic acid (both for pH adjustment)
Product Appearance and Container Contents
Eladynos is a clear and transparent injectable solution (injection). It is supplied in a cardboard box containing a multi-dose pre-filled pen with 30 doses.
Marketing Authorization Holder and Responsible for
Theramex Ireland Limited
3rd Floor, Kilmore House,
Park Lane, Spencer Dock,
Dublin 1
D01 YE64
Ireland
Manufacturing
Cilatus Manufacturing Services Ltd
Pembroke House, 28-32 Pembroke Street
Dublin, D02 EK84, Ireland
Date of Last Revision of this Leaflet:
Other Source of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu/.
----------------------------------------------------------------------------------------------------------------------------
Instructions for Use
Do not inject Eladynos until you or your caregiver have been trained by a doctor, nurse, or pharmacist on how to use the Eladynos pen.
DO NOT start the administration procedure until you have carefully read the leaflet and these instructions for use included in the Eladynos box. Always follow the instructions carefully when using the Eladynos pen.
Contact your doctor, nurse, or pharmacist if you have any questions about using the Eladynos pen.
Important Information Before Using the Eladynos Pen
- Do not share needles with other people. They can transmit a serious infection. Never share the Eladynos pen, even if you have changed the needle.
- Use a new needle for each injection.
Materials Needed for Each Injection with the Eladynos Pen
- 1 needle
Needles are not included with the Eladynos pen. The correct needles to be used with the Eladynos pen are 8 mm and 31 gauge needles.
- 1 alcohol swab
- 1 cotton ball or 1 gauze
- 1 sharps container for disposing of needles and Eladynos pens. See section 5 of the leaflet for information on disposal.
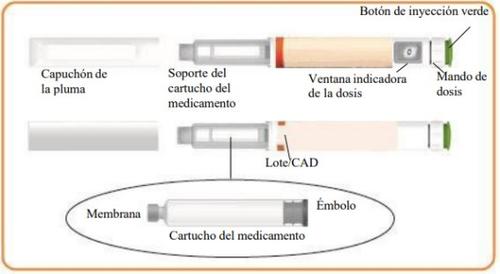
Components of the Eladynos Pen
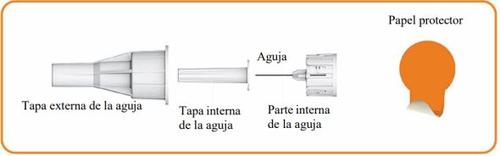
Components of the Needle (not included with the pen) to be used with the pen
How to Inject Eladynos
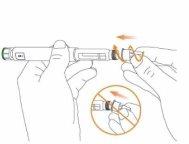
Step 1 Check the Eladynos Pen Wash your hands Check the pen labelto ensure it is the correct medication. Check the expiration date (EXP)indicated on the pen to ensure it has not expired.
Record the date of day 1 to ensure you do not use the pen for more than 30 consecutive days. Remove the pen cap.
Check that the pen, including the medication cartridge, is not damaged. The liquid should be clear and colorless and not contain visible particles; if it is not, do not use it. You may see small air bubbles in the liquid. This is normal. |
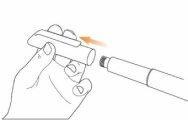
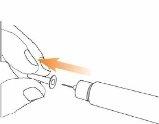
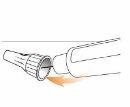
Step 2 Attach the Needle to the Eladynos Pen Remove the protective paper from a new needle.
Press the needle straightonto the pen with the cap in place and twist it until it is secure.Make sure the needle is straight so it does not bend when inserted. The pen will not work if the needle is not properly attached. Do not overtighten, as this could make it difficult to remove the needle. If the needle bends, see "Troubleshooting" later.
Remove the external needle capand set it aside for use after the injection.
Carefully remove the internal needle capand discard it.
|
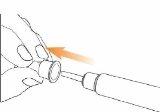
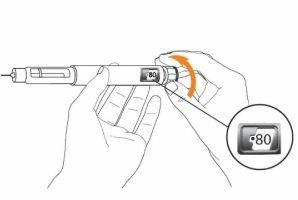
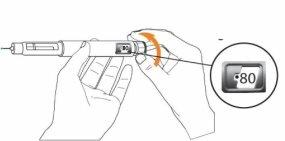
Step 3 Only on Day 1: Checking the Eladynos Pen Before the First Injection The pen contains medication for 30 days plus a small amount to test each pen once to confirm it is working correctly. Warning: If you test the pen before each injection, the medication in the pen will be depleted prematurely. Therefore, perform step 3 only on day 1, before the first injection with each pen. From day 2 to day 30, do not retest the pen; go directly to step 4 to adjust the dose for the injection. Hold the pen as shown and turn the dose button away from you until it stops. You will see "80"in the dose indicator window.
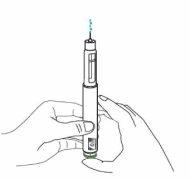
Hold the pen with the needle pointing upwards. Press the green injection button until it no longer moves. You should see liquid coming out of the needle tip, either as a drop or a stream.
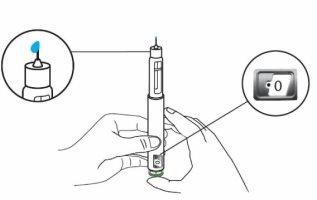
You should see "0"in the dose indicator window. Note: If no liquid comes out of the needle tip, see "Troubleshooting" later.
|
Step 4 Adjust the Dose on the Eladynos Pen Turn the white dose button away from you until it stops and "80"appears in the dose indicator window. The pen is now ready for injection.
Note: If you cannot adjust the pen to "80", see "Troubleshooting" later. |
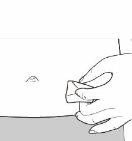
Step 5 Choose and Clean the Injection Site Injections should be administered in the lower abdomen, as shown by the gray shaded area. Avoid the area within 5 cm of the navel.
Choose a different injection site on the abdomen each day. Administer the injection only in normal skin. Do not administer the injection in areas where the skin is painful to the touch, has a bruise, or is red, scaly, or hardened. Avoid areas with scars or stretch marks. Clean the injection site with an alcohol swab and let it dry. Do not touch, ventilate, or blow on the injection site once it has been cleaned.
Note: Your doctor, nurse, or pharmacist may recommend that you pinch the skin at the injection site. Once the needle penetrates the skin, you can release the pinch. |
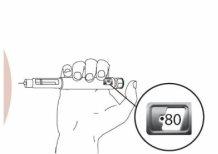
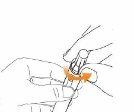
Step 6 Administer the Injection with the Eladynos Pen Insert the needle straight into the skin.
Press and HOLD the green buttonuntil ALLof the following have occurred:
and, THEN, release the button. Do not press the green button without a needle attached.
Note: Do not move the pen once inserted. If you cannot press the green injection button or it stops before "0" appears in the dose indicator window, see "Troubleshooting" later. Slowly remove the pen from the injection site by pulling straight on the needle. You may bleed slightly; this is normal. Do not rub the injection site. If there is slight bleeding, press a cotton ball or gauze against the injection site as needed. You can also cover the area with a small adhesive bandage. |
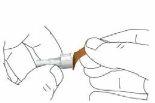
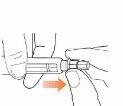
Step 7 Remove the Needle from the Pen Caution: To prevent needlestick injuries, follow this step carefully. Carefully place the external needle capback over the needle. Then, press the external needle cap until it clicks into place and is secure.
Unscrew the needle with the cap in place. To unscrew the needle with the cap in place, press the cap base against the needle and then twist it eight or more turns before gently pulling until the needle with the cap in place comes off. Note: Do not press down on the external needle cap while unscrewing the needle.
Note: You should see the space between the external needle cap and the pen increase as you unscrew the needle.
|
Step 8 After the Injection Replacethe pen capfirmly over the Eladynos pen. Keep the cap on the pen between injections.
|
Troubleshooting
What should I do if the needle bends?
- Remove the bent needle carefully and follow step 2 to attach a new needle to the pen. The pen needle has a visible part that enters the skin and an internal part that enters the pen membrane.
- Check the parts of the pen needle, paying special attention to the internal part of the needle. The visible part of the needle may appear straight, but the internal part of the needle may bend when attaching the needle to the pen.
- Make sure to keep the entire pen needle straight when attaching it to the pen to avoid bending the internal part of the needle.
What should I do if no liquid comes out of the needle tip when testing the pen on day 1?
- If you do not see liquid coming out of the needle, the pen preparation is not complete. The needle may be clogged, bent, or improperly attached.
- Follow step 2 to attach a new needle to the pen and repeat step 3 "Checking the Eladynos Pen Before the First Injection".
- If you still do not see a drop of liquid, contact your pharmacist, nurse, or doctor.
What should I do if I cannot turn the white dose button to adjust the Eladynos Pen to "80"?
- There is not enough medication in the pen to administer a full dose. You will need a new pen.
What should I do if it is difficult to press the green injection button?
- If you cannot press the green injection button or it stops before "0" appears in the dose indicator window, the new pen test is not complete. The needle may be clogged or improperly attached.
- Follow step 2 to attach a new needle to the pen.
- If you still cannot press the green injection button or it stops before "0" appears in the dose indicator window, contact your pharmacist, nurse, or doctor.
- Country of registration
- Average pharmacy price306.71 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ELADYNOS 80 micrograms/dose Injectable solution in pre-filled penDosage form: INJECTABLE, 250 micrograms/mlActive substance: teriparatideManufacturer: Gp Pharm S.A.Prescription requiredDosage form: INJECTABLE, 250 µg/mlActive substance: teriparatideManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE, 20 micrograms/80 microlitersActive substance: teriparatideManufacturer: Theramex Ireland LimitedPrescription required
Online doctors for ELADYNOS 80 micrograms/dose Injectable solution in pre-filled pen
Discuss questions about ELADYNOS 80 micrograms/dose Injectable solution in pre-filled pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions