
EKLIRA GENUAIR 322 micrograms powder for inhalation

How to use EKLIRA GENUAIR 322 micrograms powder for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information: Summary of Product Characteristics
Eklira Genuair 322micrograms inhalation powder
aclidinium (aclidinium bromide)
Read this entire leaflet carefully before you start using this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Eklira Genuair and what is it used for
- What you need to know before you use Eklira Genuair
- How to use Eklira Genuair
- Possible side effects
- Storing Eklira Genuair
- Contents of the pack and further information
Instructions for use
1. What is Eklira Genuair and what is it used for
What is Eklira Genuair
The active substance of Eklira Genuair is aclidinium bromide, which belongs to a class of medicines called bronchodilators. Bronchodilators relax the airways and help keep the bronchioles open. Eklira Genuair is a dry powder inhaler that uses your breath to deliver the medicine directly into the lungs. This makes it easier for patients with chronic obstructive pulmonary disease (COPD) to breathe.
What is Eklira Genuair used for
Eklira Genuair is indicated to help open up the airways and relieve symptoms of COPD, a serious, long-term lung disease that makes it hard to breathe. Regular use of Eklira Genuair can help you when you have ongoing difficulty breathing due to your disease, to minimize the effects of the disease on your daily life, and to reduce the number of exacerbations (worsening of COPD symptoms over several days).
2. What you need to know before you use Eklira Genuair
Do not use Eklira Genuair:
- if you are allergic to aclidinium bromide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Eklira Genuair:
- if you have heart problems;
- if you see halos around lights or colored images (glaucoma);
- if you have an enlarged prostate, problems urinating, or a blockage in your bladder.
Eklira Genuair is indicated for maintenance treatment and should not be used to treat a sudden attack of difficulty breathing or wheezing. If your COPD symptoms (difficulty breathing, wheezing, or coughing) do not improve or worsen, you should talk to your doctor as soon as possible.
Dry mouth, which has been observed with medicines like Eklira Genuair, may be associated with tooth decay after long-term use of the medicine. Therefore, remember to take care of your oral hygiene.
Stop using Eklira Genuair and seek medical help immediately if:
- you notice chest tightness, coughing, wheezing, or difficulty breathing immediately after using this medicine. These may be signs of a condition called bronchospasm.
Children and adolescents
Eklira Genuair should not be used in children or adolescents under 18 years of age.
Using Eklira Genuair with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor if you have used or are using similar medicines for respiratory problems, such as medicines containing tiotropium, ipratropium. If in doubt, consult your doctor or pharmacist. The use of Eklira Genuair with these medicines is not recommended.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. You should not use Eklira Genuair if you are pregnant or breastfeeding unless your doctor has told you to.
Driving and using machines
Eklira Genuair has a minor influence on the ability to drive and use machines. This medicine may cause headache, dizziness, or blurred vision. If you experience any of these adverse reactions, do not drive or use machines until your headache has gone, the feeling of dizziness has passed, and your vision has returned to normal.
Eklira Genuair contains lactose
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
3. How to use Eklira Genuair
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist.
The recommended dose is one inhalation twice a day, in the morning and in the evening.
The effects of Eklira Genuair last for 12 hours, so you should try to use the Eklira Genuair inhaler at the same time every morning and evening. This will ensure that there is always enough medicine in your body to help you breathe more easily throughout the day and night. This will also help you remember to use it.
The recommended dose can be used in elderly patients and in patients with kidney or liver problems. No dose adjustment is needed.
COPD is a long-term disease, so it is recommended that you use Eklira Genuair every day, twice a day, and not just when you have breathing problems or other COPD symptoms.
Method of administration
This medicine is for inhalation use only.
Read the Instructions for Use to learn how to use the Genuair inhaler. If you are in doubt about how to use Eklira Genuair, consult your doctor or pharmacist.
You can use Eklira Genuair at any time, before or after meals or drinks.
If you use more Eklira Genuair than you should
If you think you have used more Eklira Genuair than you should, talk to your doctor or pharmacist.
If you forget to use Eklira Genuair
If you miss a dose of Eklira Genuair, inhale the dose as soon as you remember. However, if it is almost time for your next dose, skip the missed dose.
Do not take a double dose to make up for a forgotten dose.
If you stop using Eklira Genuair
This medicine is for long-term treatment. If you want to stop treatment, talk to your doctor first, as your symptoms may get worse.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Eklira Genuair can cause side effects, although not everybody gets them.
Rarely, allergic reactions (may affect up to 1 in 1,000 patients) can occur. Stop using the medicine and contact your doctor immediately if you experience swelling of the face, throat, lips, or tongue (with or without difficulty breathing or swallowing), dizziness or fainting, rapid heartbeat, or severe swelling with itching of the skin (hives), as these may be symptoms of an allergic reaction.
The following side effects may occur when using Eklira Genuair:
Common:may affect up to 1 in 10 patients
- Headache
- Inflammation of the sinuses (sinusitis)
- Common cold (nasopharyngitis)
- Cough
- Diarrhea
- Nausea
Uncommon:may affect up to 1 in 100 patients
- Dizziness
- Dry mouth
- Inflammation of the mouth (stomatitis)
- Hoarseness (dysphonia)
- Rapid heartbeat (tachycardia)
- Feeling of heart pounding (palpitations)
- Abnormal or irregular heartbeat (arrhythmias)
- Difficulty urinating (urinary retention)
- Blurred vision
- Rash
- Itching of the skin
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Eklira Genuair
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the inhaler label and the carton after “EXP”. The expiry date is the last day of the month stated.
Keep the Genuair inhaler in the bag until you are ready to start using it.
Use within 90 days of opening the bag.
Do not use Eklira Genuair if you notice the packaging is damaged or shows signs of tampering.
Once you have used all the doses, the inhaler should be disposed of. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Eklira Genuair Composition
- The active ingredient is aclidinium bromide. Each delivered dose contains 375 micrograms of aclidinium bromide equivalent to 322 micrograms of aclidinium.
- The other component is lactose monohydrate (see section 2 "Eklira Genuair contains lactose").
Product Appearance and Container Contents
Eklira Genuair is a white or almost white powder.
The Genuair inhaler device is white with an integrated dose indicator and a green dosing button. The mouthpiece is covered by a removable green protective cap. It is provided in a plastic bag.
Available container sizes:
Container containing 1 inhaler with 30 doses.
Container containing 1 inhaler with 60 doses.
Container containing 3 inhalers with 60 doses each.
Only some container sizes may be marketed.
Marketing Authorization Holder
Covis Pharma Europe B.V.
Gustav Mahlerplein 2
1082MA Amsterdam
Netherlands
Manufacturer
Industrias Farmacéuticas Almirall, S.A.
Ctra. de Martorell 41-61
08740 Sant Andreu de la Barca, Barcelona
Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Covis Pharma Europe B.V. Tel: 80013067 | Lietuva Covis Pharma Europe B.V. Tel: 880000890 |
| Luxembourg/Luxemburg Covis Pharma Europe B.V. Tel: 80024119 |
Ceská republika Covis Pharma Europe B.V. Tel: 800144474 | Magyarország Covis Pharma Europe B.V. Tel.: 0680021540 |
Danmark Zentiva Denmark ApS Tlf: +45 787 68 400 | Malta Covis Pharma Europe B.V. Tel: 80065149 |
Deutschland Zentiva Pharma GmbH Tel: +49 (0) 800 53 53 010 | Nederland Covis Pharma Europe B.V. Tel: 08000270008 |
Eesti Covis Pharma Europe B.V Tel: 8000100776 | Norge Zentiva Denmark ApS Tlf: +47 219 66 203 |
Ελλáδα Specialty Therapeutics IKE Τηλ: +30 213 02 33 913 | Österreich Covis Pharma Europe B.V. Tel: 0800006573 |
España Zentiva Spain S.L.U. Tel: +34 931 815 250 | Polska Covis Pharma Europe B.V. Tel.: 0800919353 |
France Zentiva France Tél: +33 (0) 800 089 219 | Portugal Zentiva Portugal, Lda Tel: +351210601360 |
Hrvatska Covis Pharma Europe B.V. Tel: 08004300 | România Covis Pharma Europe B.V. Tel: 0800410175 |
Ireland Zentiva Denmark ApS Sími: +354 539 0650 | Slovenija Covis Pharma Europe B.V. Tel: 080083003 |
Ísland Zentiva Denmark ApS Sími: +354 539 0650 | Slovenská republika Covis Pharma Europe B.V. Tel: 0800008203 |
Italia Covis Pharma Europe B.V. Tel: 800168094 | Suomi/Finland Zentiva Denmark ApS Puh/Tel: +358 942 598 648 |
Κúπρος Specialty Therapeutics IKE Τηλ: +30 213 02 33 913 | Sverige Zentiva Denmark ApS Tel: +46 840 838 822 |
Latvija Covis Pharma Europe B.V. Tel: 80005962 | United Kingdom (Northern Ireland) Zentiva, k.s. Tel: +44 (0) 800 090 2408 |
Date of Last Revision of this Leaflet:
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for Use
This section contains information on how to use your Genuair inhaler. It is important that you read this information, as Genuair may work differently from inhalers you have used before. If you have any questions about how to use your inhaler, ask your doctor, pharmacist, or nurse for help.
The instructions for use are divided into the following sections:
- Getting Started
- Step 1: Prepare your dose
- Step 2: Inhale your medication
- Additional Information
Getting Started
Read these instructions for use before starting to use the medication
Familiarize yourself with the parts of your Genuair inhaler
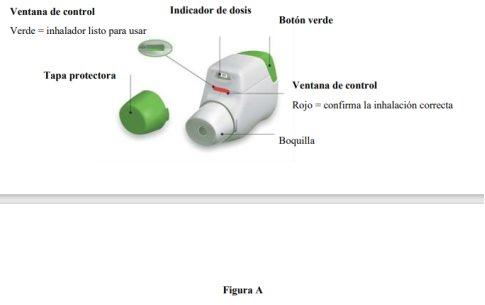
Before Use:
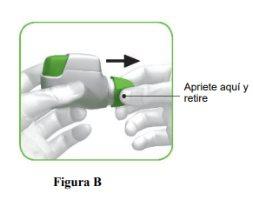
- Before the first use, open the sealed bag and remove the inhaler. Dispose of the bag and desiccant.
- Do not press the orange button until you are ready to inhale a dose.
- Remove the cap by gently pressing the arrows on either side (Figure B).
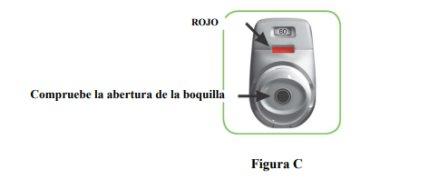
STEP 1: Prepare your dose
- Check the mouthpiece opening and make sure nothing is blocking it (Figure C).
- Check the control window (it should be red, Figure C).
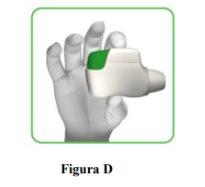
- Hold the inhaler horizontally with the mouthpiece facing you and the orange button on top (Figure D).
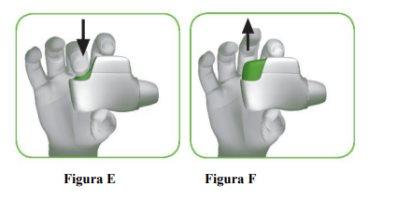
- Press the orange button down and all the way to load your dose (Figure E).
When you press the orange button down and all the way, the control window will change from red to green.
Make sure the orange button is back up. Do not tilt it. |
- Release the orange button (Figure F).
Make sure to release the button so the inhaler can work correctly. |
Stop and Check:
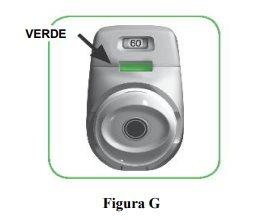
- Make sure the control window is now green (Figure G).
Your medication is ready to be inhaled.
Go to “STEP 2: Inhale your medication”.
What to do if the control window remains red after pressing the button (Figure H).
The dose is not prepared. Go back to “STEP 1 Prepare your dose” and repeat steps 1.1 to 1.6. |
STEP 2: Inhale your medication
Read steps 2.1 to 2.7 completely before using. Do not tilt. |
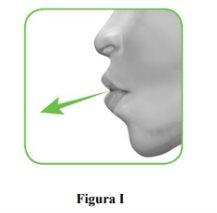
2.1 Keep the inhaler away from your mouth and exhale completely.Never exhale into the inhaler (Figure I).
2.2 Keep your head upright, place the mouthpiece between your lips, and close them tightly around the mouthpiece (Figure J).
Do not keep the orange button pressed while inhaling. |
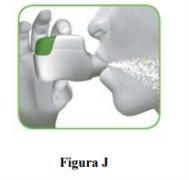
2.3 Take a strong and deep breaththrough your mouth. Keep breathing in for as long as possible.
A “click” will let you know that you have inhaled correctly. Keep breathing in for as long as possible after hearing the “click”. Some patients may not hear the “click”. Use the control window to make sure you have inhaled correctly. |
2.4 Remove the inhaler from your mouth.
2.5 Hold your breath for as long as possible.
2.6 Breathe out slowly away from the inhaler.
Some patients may experience a gritty sensation in the mouth or a slightly sweet or bitter taste. Do not inhale an extra dose if you do not notice any taste or do not feel anything after inhaling. |
Stop and Check:
2.7 Make sure the control window is now red (Figure K). This means you have inhaled your medication correctly.

What to do if the control window remains green after inhaling (Figure L).
This means you have not inhaled your medication correctly. Go back to “STEP 2 Inhale your medication” and repeat steps 2.1 to 2.7. If the control window still does not change to red, you may have forgotten to release the orange button before inhaling, or you may not have inhaled strongly enough. If this happens, try again. Make sure you have released the orange button and that you have exhaled completely. Then take a strong and deep breath through the mouthpiece. Please contact your doctor if the control window remains green after several attempts. |
Put the protective cap back on the mouthpiece after each use (Figure M), to prevent contamination of the inhaler with dust or other materials. You must discard your inhaler if you lose the cap.

Additional Information:
What should you do if you accidentally load a dose?
Keep your inhaler with the protective cap in place until it is time to inhale your medication, then remove the cap and start at step 1.6.
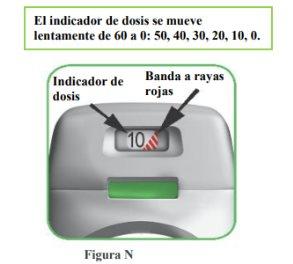
How does the dose indicator work?
- The dose indicator shows the total number of doses left in the inhaler (Figure N).
- At the first use, each inhaler contains at least 60 or 30 doses, depending on the container size.
- Each time a dose is loaded by pressing the orange button, the dose indicator moves slightly to the next number (50, 40, 30, 20, 10, or 0).
When should you get a new inhaler?:
You should get a new inhaler:
- If your inhaler appears to be damaged or loses its cap, or
- When a red striped band appears on the dose indicator, this means you are approaching the last dose (Figure N), or
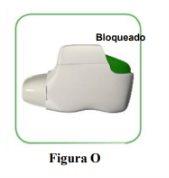
- If your inhaler is empty (Figure O).
How do you know if your inhaler is empty?:
When the orange button does not return to its fully up position and remains stuck in a middle position, it has reached the last dose (Figure O). Even when the orange button is stuck, you can still inhale the last dose. After that, the inhaler cannot be used again and you should start using a new inhaler.
How should you clean the inhaler?
NEVER use water to clean the inhaler as it could damage your medication.
If you want to clean your inhaler, simply wipe the outside of the mouthpiece with a dry cloth or paper towel.
- Country of registration
- Average pharmacy price47.61 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EKLIRA GENUAIR 322 micrograms powder for inhalationDosage form: PULMONARY INHALATION, 322 microgramsActive substance: aclidinium bromideManufacturer: Covis Pharma Europe B.V.Prescription requiredDosage form: PULMONARY INHALATION, 0.0040 g/aerosolActive substance: ipratropium bromideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 20 µgActive substance: ipratropium bromideManufacturer: Boehringer Ingelheim Espana S.A.Prescription required
Online doctors for EKLIRA GENUAIR 322 micrograms powder for inhalation
Discuss questions about EKLIRA GENUAIR 322 micrograms powder for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions


















