EBGLYSS 250 MG PRE-FILLED PEN INJECTION SOLUTION

How to use EBGLYSS 250 MG PRE-FILLED PEN INJECTION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ebglyss 250mg solution for injection in pre-filled pen
lebrikizumab
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ebglyss and what is it used for
- What you need to know before you use Ebglyss
- How to use Ebglyss
- Possible side effects
- Storage of Ebglyss
- Contents of the pack and other information
Instructions for use
1. What is Ebglyss and what is it used for
Ebglyss contains the active substance lebrikizumab.
Ebglyss is used to treat adults and adolescents from 12 years of age with moderate to severe atopic dermatitis (also known as atopic eczema) who can be treated with systemic therapies (medicines given by mouth or by injection).
Ebglyss can be given alone or in combination with medicines for eczema that are applied to the skin.
Lebrikizumab is a monoclonal antibody (a type of protein) that blocks the action of another protein called interleukin 13. Interleukin 13 plays an important role in the symptoms of atopic dermatitis. By blocking interleukin 13, Ebglyss can improve your atopic dermatitis and reduce the itching and skin pain associated with it.
2. What you need to know before you use Ebglyss
Do not use Ebglyss
- if you are allergic to lebrikizumab or any of the other ingredients of this medicine (listed in section 6).
If you think you may be allergic or are unsure, talk to your doctor, pharmacist, or nurse before using Ebglyss.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Ebglyss.
Each time you get a new pack of Ebglyss, it is important that you note the batch number (found on the pack after “Batch”) and that you keep this information in a safe place.
Allergic reactions
Very rarely, this medicine can cause allergic reactions (hypersensitivity). These reactions can occur soon after starting Ebglyss but can also occur later. If you notice that you have symptoms of an allergic reaction, you must stop using the medicine and contact your doctor or get medical attention immediately. The signs of an allergic reaction include:
- breathing problems
- swelling of the face, mouth, and tongue
- fainting
- dizziness
- feeling faint (due to a drop in blood pressure)
- hives, itching, and skin rash.
Eye problems
Talk to your doctor if you notice any new or worsening eye problems, such as redness and discomfort in the eye, eye pain, or changes in vision.
Vaccines
Talk to your doctor about your current vaccination program. See the section “Other medicines and Ebglyss”.
Children and adolescents
This medicine must not be used in children with atopic dermatitis under 12 years of age or in adolescents from 12 to 17 years of age and weighing less than 40 kg, as it has not been studied in this age group.
Other medicines and Ebglyss
Tell your doctor or pharmacist if:
- you are using, have recently used, or might use any other medicines;
- you have recently been vaccinated or are planning to be vaccinated. You must not receive certain types of vaccines (live vaccines) during treatment with Ebglyss.
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
The effects of this medicine in pregnant women are not known; it is best to avoid using Ebglyss during pregnancy unless your doctor advises you to use it.
It is not known whether lebrikizumab passes into breast milk. If you are breast-feeding or plan to breast-feed, talk to your doctor before using this medicine. You and your doctor will decide whether to breast-feed or use Ebglyss. You should not do both.
Driving and using machines
Ebglyss is unlikely to affect your ability to drive or use machines.
3. How to use Ebglyss
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, talk to your doctor or pharmacist again.
How much Ebglyss to use and for how long
Your doctor will decide how much Ebglyss you need and for how long you will use it.
The recommended dose is:
- Two initial injections of 250 mg of lebrikizumab each (500 mg in total) at week 0 and week 2.
- One injection with 250 mg every two weeks from week 4 to week 16.
Depending on how you respond to the medicine, your doctor may decide to stop giving you the medicine or continue giving you one injection of 250 mg every two weeks up to week 24.
- One injection with 250 mg every four weeks from week 16 onwards (maintenance posology).
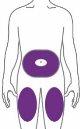
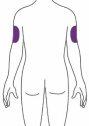
Ebglyss is given by injection under the skin (subcutaneously) in the thigh or abdomen, except in the 5 cm around the navel. If someone else gives you the injection, it can also be given in the upper arm. You and your doctor or nurse will decide if you can inject Ebglyss yourself.
It is recommended that you alternate the injection site with each injection. Ebglyss must not be injected into sensitive skin areas, damaged skin, or skin with bruises or scars, or into skin areas affected by atopic dermatitis or other skin lesions. The initial dose of 500 mg should be administered in two consecutive injections of 250 mg at different injection sites.
It is important that you do not try to give yourself the injection until your doctor or nurse has taught you how to do it. It is also possible that a caregiver who has learned to do it well will give you the Ebglyss injections. In the case of adolescents, 12 years of age or older, it is recommended that Ebglyss be administered by an adult or under the supervision of an adult.
The pre-filled pen must not be shaken.
Read the “Instructions for use” of the pre-filled pen carefully before administering Ebglyss.
If you use more Ebglyss than you should
If you use more Ebglyss than your doctor prescribed or if you inject a dose earlier than planned, talk to your doctor, pharmacist, or nurse.
If you forget to use Ebglyss
If you have forgotten to inject a dose of Ebglyss, talk to your doctor, pharmacist, or nurse.
If you forgot to inject Ebglyss when you usually do, inject the dose when you remember. The next dose should be injected at the usual scheduled time.
If you stop using Ebglyss
Do not stop using Ebglyss without talking to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common(may affect up to 1 in 10 people)
- Redness and discomfort in the eye (conjunctivitis)
- Inflammation of the eye due to an allergic reaction (allergic conjunctivitis)
- Dry eye
- Reactions at the injection site
Uncommon(may affect up to 1 in 100 people)
- Herpes zoster (shingles), a painful rash with blisters on one part of the body
- Increased eosinophils (a type of white blood cell, eosinophilia)
- Inflammation of the cornea (the transparent layer covering the front of the eye, keratitis)
- Itching, redness, and swelling of the eyelids (blepharitis)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Agency's website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ebglyss
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label after EXP. The expiry date refers to the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Store in the original package to protect from light.
Do not use this medicine if you notice that the solution is cloudy or has changed color or if it contains visible particles. Before use, remove the carton from the refrigerator, remove the pre-filled pen from the carton, and wait 45 minutes to reach room temperature. After removal from the refrigerator, Ebglyss must be stored below 30°C and used within 7 days or discarded. Once stored at room temperature, it must not be refrigerated again. You can write the date you removed it from the refrigerator on the carton.
This medicine is for single use only.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Ebglyss Composition
- The active ingredient is lebrikizumab. Each pre-filled pen contains 250 mg of lebrikizumab in 2 ml of solution (125 mg/ml).
- The other components are histidine, glacial acetic acid (E260), sucrose, polysorbate 20 (E432), and water for injectable preparations.
Product Appearance and Container Contents
Ebglyss is a sterile injectable solution that is clear to opalescent, colorless to slightly yellow or slightly brown, and free of visible particles. It is supplied in cartons containing one single-dose pre-filled pen or two single-dose pre-filled pens, and in multiple packs containing three single-dose pre-filled pens (3 packs of 1), four single-dose pre-filled pens (2 packs of 2), five single-dose pre-filled pens (5 packs of 1), or six single-dose pre-filled pens (3 packs of 2). Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Almirall, S.A.
Ronda General Mitre, 151
08022 Barcelona
Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Spain
Almirall, S.A.
Tel: +34 93 291 30 00
Date of Last Revision of this Leaflet: 11/2023.
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
Instructions for Use
These Instructions for Use contain information on how to administer Ebglyss injections. |
Read these "Instructions for Use" before using this medication and carefully follow all the step-by-step instructions. |
|
Important Information You Need to Know Before Injecting Ebglyss |
|
|
|
|
|
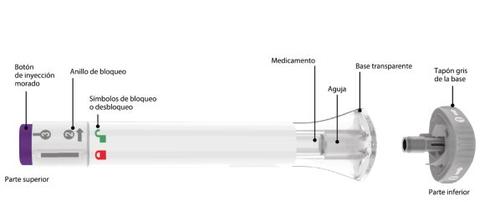
Parts of the Ebglyss Pre-filled Pen |
|
Preparation for Ebglyss Injection | ||
Prepare the supplies: | ||
|
| |
Wait 45 minutes | ||
Remove the Ebglyss pre-filled pen from the carton with the gray cap on the base and let the pre-filled pen reach room temperature for 45 minutes before injection.
| ||
Inspect the Pre-filled Pen and Medication | ||
Make sure you have the correct medication. The medication inside the pre-filled pen should be clear. It may be colorless to slightly yellow or slightly brown. | ||
| Do notuse the pre-filled pen (see the section Disposal of the Ebglyss Pre-filled Pen) if:
| |
Wash your hands with water and soap | ||
Select and Clean the Injection Site | ||
Your doctor may help you choose the injection site that is most convenient for you. Clean the injection site with an alcohol swab and let it dry. | ||
| You or another person may inject the medication, or it may be injected by someone else, in these areas. |
At least 5 cm from the navel.
At least 5 cm above the knee and 5 cm below the groin. |
| Someone else should inject the medication in this area. |
Someone else should inject into the outer aspect of the arm. Do notinject in the same spot every time. Do not injectin areas of sensitive skin, with bruises, redness, hardness, or scarring, or in areas of skin affected by atopic dermatitis or other skin lesions. |
Ebglyss Injection | ||||
1 | Remove the Cap from the Pre-filled Pen |
| ||
| Make sure the pre-filled pen is locked. | |||
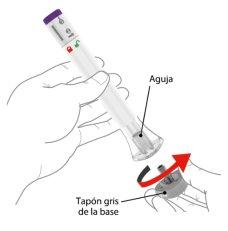
When you are ready to administer the injection, twist the gray base cap and throw it away in the household trash. Do notreplace the gray base cap, as it may damage the needle. Do nottouch the needle inside the transparent base. | ||||
2 | Place and Unlock | |||
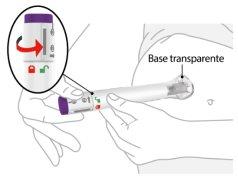
| Placethe transparent base against the skin and hold it firmly. | |||
Keep the transparent base on the skin and then twist the lock ring to the unlockposition. | ||||
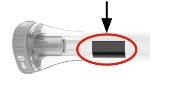
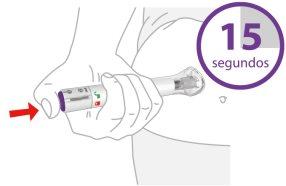
3 | Press and Hold for 15 Seconds | |||
| Pressthe purple injection button, hold it down, and wait to heartwo loud clicks:
The injection may take up to 15 seconds. | |||
You will know that the injection is complete when the gray plunger is visible. Then, remove the pre-filled pen from the injection site. |
| Gray Plunger |
Disposal of the Ebglyss Pre-filled Pen | |
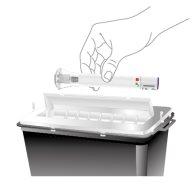
Dispose of the Used Pre-filled Pen | |
| Dispose of the used Ebglyss pre-filled pen in a sharps container immediately after use. |
Do notthrow the Ebglyss pre-filled pen in the household trash. | |
If you do not have a sharps container, you can use a household container that:
| |
When the sharps container is almost full, you will need to follow your local guidelines for its proper disposal. There may be national laws regarding the disposal of needles and syringes. For more information on the safe disposal of sharps, ask your doctor about the options available in your area. Do notrecycle the used sharps container. | |
Frequently Asked Questions | |
Q. | What happens if there are bubbles in the pre-filled pen? |
A. | It is normal to have air bubbles. They will not harm you or affect your dose. |
Q. | What happens if there is a drop of liquid on the needle tip when I remove the gray base cap? |
A. | It is normal to have a drop of liquid on the needle tip. It will not harm you or affect your dose. |
Q. | What happens if I unlock the pre-filled pen and press the purple injection button before twisting the gray base cap? |
A. | Do notremove the gray base cap. Discard (throw away) the pre-filled pen and use a new one. |
Q. | Should I keep pressing the injection button until the injection is complete? |
A. | It is not necessary to keep pressing the injection button, but it may help you keep the pre-filled pen stable and firm against the skin. |
Q. | What happens if the needle does not retract after the injection? |
A. | Do nottouch the needle or replace the gray base cap. Store the pre-filled pen in a safe place to avoid an accidental needle stick. |
Q. | What happens if I have a drop of liquid or blood on the skin after the injection? |
A. | This is normal. Apply pressure to the injection site with a cotton ball or gauze. Do notrub the injection site. |
Q. | How do I know if my injection is complete? |
A. | After pressing the purple injection button, you will hear 2 loud clicks. The second loud click indicates that the injection is complete. You will also see the gray plunger in the top of the transparent base. The injection may take up to 15 seconds. |
Q. | What happens if I remove the pre-filled pen before the second click or before the gray plunger has stopped moving? |
A. | You may not have received the full dose. Do not administer another injection. Call your doctor for help. |
Q. | What happens if I hear more than 2 clicks during the injection? (2 loud clicks and 1 soft click). Did I receive the full injection? |
A. | Some people may hear a soft click just before the second loud click. This is the normal operation of the pre-filled pen. Do notremove the pre-filled pen from the skin until you hear the second loud click. |
Read the complete leaflet of the pre-filled pen before using Ebglyss.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EBGLYSS 250 MG PRE-FILLED PEN INJECTION SOLUTIONDosage form: INJECTABLE, 250 mgActive substance: lebrikizumabManufacturer: Almirall S.A.Prescription requiredDosage form: INJECTABLE, 1 mlActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription requiredDosage form: INJECTABLE, 300 mgActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription required
Online doctors for EBGLYSS 250 MG PRE-FILLED PEN INJECTION SOLUTION
Discuss questions about EBGLYSS 250 MG PRE-FILLED PEN INJECTION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions