
DUPIXENT 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use DUPIXENT 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Dupixent 300 mg solution for injection in pre-filled syringe
dupilumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Dupixent and what is it used for
- What you need to know before you use Dupixent
- How to use Dupixent
- Possible side effects
- Storing Dupixent
- Contents of the pack and other information
1. What is Dupixent and what is it used for
What is Dupixent
Dupixent contains the active substance dupilumab.
Dupilumab is a monoclonal antibody (a type of specialized protein) that blocks the action of proteins called interleukins (IL)-4 and IL-13. Both play a key role in the appearance of signs and symptoms of atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP), nodular prurigo (NP), eosinophilic esophagitis (EoE), and chronic obstructive pulmonary disease (COPD).
What is it used for
Dupixent is used to treat adults and adolescents from 12 years of age with moderate to severe atopic dermatitis, also known as atopic eczema. Dupixent is also used to treat children from 6 months to 11 years of age with severe atopic dermatitis. Dupixent can be used with medications for eczema that are applied to the skin or can be used alone.
Dupixent is also used, along with other medications for asthma, for the maintenance treatment of severe asthma in adults, adolescents, and children from 6 years of age whose asthma is not controlled with their current medication (e.g., corticosteroids).
Dupixent is also used, along with other medications, for the maintenance treatment of CRSwNP in adults whose disease is not controlled with their current medication for CRSwNP. Dupixent may also reduce the need for surgery and the need for systemic corticosteroids.
Dupixent is used to treat adults with moderate to severe nodular prurigo (NP), also known as chronic nodular prurigo (CNP). Dupixent can be used with medications for NP that are applied to the skin or can be used alone.
Dupixent is used to treat adults, adolescents, and children from 1 year of age, with a minimum weight of 15 kg, with eosinophilic esophagitis (EoE).
Dupixent is used, along with other medications, for the maintenance treatment of chronic obstructive pulmonary disease (COPD) in adults with uncontrolled COPD.
How Dupixent works
Using Dupixent for atopic dermatitis (atopic eczema) may improve your skin disease and reduce itching. Dupixent has also shown an improvement in symptoms of pain, anxiety, and depression associated with atopic dermatitis. Additionally, Dupixent helps improve sleep disorders and your overall quality of life.
Dupixent helps prevent severe asthma attacks (exacerbations) and may improve your breathing ability. Dupixent may also help reduce the amount of another group of medications you need to control your asthma, called oral corticosteroids, while preventing severe asthma attacks and improving your breathing ability.
Dupixent helps prevent moderate or severe COPD attacks (exacerbations) and may improve your breathing ability. Dupixent may also help improve the overall symptoms of COPD.
2. What you need to know before you use Dupixent
Do not use Dupixent
If you think you may be allergic or are not sure, consult your doctor, pharmacist, or nurse before using Dupixent.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting Dupixent:
Dupixent is not a rescue medicineand should not be used to treat a sudden asthma or COPD attack.
Each time you have a new pack of Dupixent, it is important that you note the name of the medicine, the date of administration, and the batch number (which is found on the packaging after “Batch”) and keep this information in a safe place.
Allergic reactions
- Rarely, Dupixent may cause serious side effects, including allergic reactions (hypersensitivity), anaphylactic reaction, and angioedema. These reactions can occur from minutes after Dupixent administration to seven days after administration. While using Dupixent, you should watch for signs of these diseases (i.e., breathing problems, swelling of the face, lips, mouth, throat, or tongue, fainting, dizziness, feeling of dizziness (low blood pressure), fever, feeling of discomfort, swelling of the lymph nodes, hives, itching, joint pain, skin rash). These signs are listed in "Serious side effects" in section 4.
- Stop using Dupixent and tell your doctor or get medical help immediately if you notice any sign of an allergic reaction.
Eosinophilic diseases
- Rarely, patients taking an asthma medication may develop inflammation of the blood vessels or lungs as a result of an increase in a certain type of white blood cell (eosinophilia).
- It is not known if this is caused by Dupixent. This usually, but not always, occurs in people who are also taking a steroid medication that has been discontinued or is being reduced in dose.
- If you experience a combination of symptoms that include a flu-like illness, tingling or numbness of the arms or legs, worsening of lung symptoms, and/or rash, inform your doctor immediately.
Parasitic infection (intestinal parasites)
- Dupixent may weaken your resistance to parasitic infections. If you already have a parasitic infection, it should be treated before starting treatment with Dupixent.
- Consult your doctor if you have diarrhea, gas, stomach discomfort, greasy stools, and dehydration that may be a sign of a parasitic infection.
- If you live in a region where these infections are common or if you are traveling to that region, consult your doctor.
Asthma
If you have asthma and are taking asthma medication, do not change or stop taking your asthma medication without consulting your doctor. Consult your doctor before stopping treatment with Dupixent or if your asthma is not controlled or worsens during treatment with this medicine.
Eye problems
Consult your doctor if eye problems appear or worsen, including eye pain or changes in vision.
Children and adolescents
- The safety and benefits of Dupixent in children under 6 months of age with atopic dermatitis are not yet known.
- The safety and benefits of Dupixent in children under 6 years of age with asthma are not yet known.
- The safety and benefits of Dupixent in children under 18 years of age with CRSwNP are not yet known.
- The safety and benefits of Dupixent in children under 18 years of age with NP are not yet known.
- The safety and benefits of Dupixent in children under 1 year of age, or with a body weight <15 kg with eoe, are not yet known.< li>
- The safety and benefits of Dupixent in children under 18 years of age with COPD are not yet known.
Other medicines and Dupixent
Tell your doctor or pharmacist
Other asthma medicines
Do not stop or reduce your asthma medicines unless your doctor tells you to.
- These medicines (especially those called corticosteroids) should be stopped gradually.
- This should be done under the direct supervision of your doctor and depending on your response to Dupixent.
Pregnancy and breastfeeding
- If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. The effects of this medicine in pregnant women are not known; therefore, it is preferable to avoid using Dupixent in pregnancy unless your doctor advises you to do so.
- If you are breastfeeding or plan to breastfeed, consult your doctor before using this medicine. Your doctor and you must decide whether to breastfeed or use Dupixent. You should not do both.
Driving and using machines
It is unlikely that Dupixent will affect your ability to drive or use machines.
Dupixent contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 300 mg dose; this is essentially “sodium-free”.
Dupixent contains polysorbate
This medicine contains 4 mg of polysorbate 80 in each 300 mg dose (2 ml). Polysorbates may cause allergic reactions. Tell your doctor if you or your child have any known allergy.
3. How to use Dupixent
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist.
What dose of Dupixent you will receive
Your doctor will decide what dose of Dupixent is suitable for you.
Recommended dose in adults with atopic dermatitis
For patients with atopic dermatitis, the recommended dose of Dupixent is:
- An initial dose of 600 mg (two 300 mg injections)
- Followed by 300 mg every two weeks by subcutaneous injection.
Recommended dose in adolescents with atopic dermatitis
The recommended dose of Dupixent for adolescents (12 to 17 years of age) with atopic dermatitis is based on body weight:
Patient body weight | Initial dose | Subsequent doses (every two weeks) |
less than 60 kg | 400 mg (two 200 mg injections) | 200 mg |
60 kg or more | 600 mg (two 300 mg injections) | 300 mg |
Recommended dose in children from 6 to 11 years of age with atopic dermatitis
The recommended dose of Dupixent for children (6 to 11 years of age) with atopic dermatitis is based on body weight:
Patient body weight | Initial dose | Subsequent doses |
15 kg to less than 60 kg | 300 mg (one 300 mg injection) on day 1, followed by 300 mg on day 15 | 300 mg every 4 weeks*, starting 4 weeks after the day 15 dose |
60 kg or more | 600 mg (two 300 mg injections) | 300 mg every two weeks |
- The dose may be increased to 200 mg every two weeks as advised by your doctor.
Recommended dose in children from 6 months to 5 years of age with atopic dermatitis
The recommended dose of Dupixent for children from 6 months to 5 years of age with atopic dermatitis is based on body weight:
Patient body weight | Initial dose | Subsequent doses |
5 kg to less than 15 kg | 200 mg (one 200 mg injection) | 200 mg every 4 weeks |
15 kg to less than 30 kg | 300 mg (one 300 mg injection) | 300 mg every 4 weeks |
Recommended dose in patients with asthma (from 12 years of age)
For patients with severe asthma and taking oral corticosteroids or for patients with severe asthma and moderate to severe comorbid atopic dermatitis, the recommended dose of Dupixent is:
- An initial dose of 600 mg (two 300 mg injections)
- Followed by 300 mg administered every two weeks by subcutaneous injection.
For the rest of the patients with severe asthma, the recommended dose of Dupixent is:
- An initial dose of 400 mg (two 200 mg injections)
- Followed by 200 mg administered every two weeks by subcutaneous injection.
Recommended dose in children with asthma
The recommended dose of Dupixent for children (6 to 11 years of age) with asthma is based on body weight:
Patient body weight | Initial and subsequent doses |
15 kg to less than 30 kg | 300 mg every 4 weeks |
30 kg to less than 60 kg | 200 mg every two weeks or 300 mg every 4 weeks |
60 kg or more | 200 mg every two weeks |
For patients 6 to 11 years of age with asthma and severe coexisting atopic dermatitis, your doctor will decide what dose of Dupixent is suitable for you.
Recommended dose in adults with chronic rhinosinusitis with nasal polyposis (CRSwNP)
In CRSwNP, the first recommended dose of Dupixent is 300 mg followed by 300 mg every two weeks by subcutaneous injection.
Recommended dose in adults with nodular prurigo (NP)
For patients with nodular prurigo, the recommended dose of Dupixent is:
- An initial dose of 600 mg (two 300 mg injections)
- Followed by 300 mg administered every two weeks by subcutaneous injection.
Recommended dose in adults, adolescents, and children (from 1 year of age) with eosinophilic esophagitis (EoE)
Body weight | Dose |
≥15 kg to <30 kg< p> | 200 mg every two weeks |
≥30 kg to <40 kg< p> | 300 mg every two weeks |
≥40 kg | 300 mg every week |
Recommended dose in adults with chronic obstructive pulmonary disease (COPD)
In COPD, the recommended dose of Dupixent is 300 mg administered every two weeks by subcutaneous injection.
Dupixent injection
Dupixent is administered by injection under your skin (subcutaneous injection). Your doctor or nurse and you must decide if you should inject Dupixent yourself.
Before you inject Dupixent yourself, you must have been properly trained by your doctor or nurse. Your Dupixent injection can also be administered by a caregiver after proper training by a doctor or nurse. Each pre-filled syringe contains one dose of Dupixent (300 mg). Do not shake the pre-filled syringe. Carefully read the “Instructions for Use” included at the end of the package leaflet before using Dupixent.
If you use more Dupixent than you should
If you use more Dupixent than you should or have been given the dose too soon, consult your doctor, pharmacist, or nurse.
If you forget to use Dupixent
If you have forgotten to inject a dose of Dupixent, consult your doctor, pharmacist, or nurse.
Also,
If your dosing schedule is every weekand you forget a dose of Dupixent:
- administer the Dupixent injection as soon as possible and start a new weekly dosing schedule from the time you remember to administer your Dupixent injection.
If your dosing schedule is every two weeksand you forget a dose of Dupixent:
- administer the Dupixent injection within 7 days of the missed dose, then follow your original schedule.
If your dosing schedule is every 4 weeksand you forget a dose of Dupixent:
- administer the Dupixent injection within 7 days of the missed dose, then follow your original schedule.
If you stop treatment with Dupixent
Do not stop treatment with Dupixent without discussing it with your doctor first.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine may cause adverse effects, although not all people suffer from them.
Dupixent may cause serious adverse effects, including rare allergic reactions (hypersensitivity), including anaphylactic reaction, serum sickness, serum sickness-like reaction; the signs may include:
- breathing problems
- swelling of the face, lips, mouth, throat, or tongue (angioedema)
- fainting, dizziness, feeling of dizziness (low blood pressure)
- fever
- feeling of general discomfort
- inflammation of the lymph nodes
- hives
- itching
- joint pain
- skin rash
If you develop an allergic reaction, stop using Dupixent and consult your doctor immediately.
Other Adverse Effects
Frequent(may affect up to 1 in 10 people):
- reactions at the injection site (e.g., localized redness, swelling, itching, pain, bruising)
- redness and itching of the eyes
- eye infection
- herpes (on the lips and skin)
- an increase in a certain number of white blood cells (eosinophils)
- joint pain (arthralgia)
Uncommon(may affect up to 1 in 100 people):
- swelling of the face, lips, mouth, throat, or tongue (angioedema)
- itching, redness, and swelling of the eyelids
- inflammation of the surface of the eye, sometimes with blurred vision (keratitis)
- facial rash or redness
- dry eyes
Rare(may affect up to 1 in 1,000 people):
- severe allergic reactions (hypersensitivity)
- ulcers on the transparent outer layer of the eye, sometimes with blurred vision (ulcerative keratitis)
Additional Adverse Effects in Children from 6 to 11 Years with Asthma
Frequent: worms (enterobiasis)
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Dupixent
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after EXP. The expiry date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze. Keep in the original package to protect from light.
If necessary, the pre-filled syringe can be removed from the refrigerator and stored in the carton for a maximum of 14 days at room temperature up to 25°C, protected from light. The date it is removed from the refrigerator will be noted in the space provided for this purpose on the outer carton. The carton must be discarded if it is left out of the refrigerator for more than 14 days or if the expiry date has passed.
Do not use this medicine if you notice that the medicine is cloudy, discolored, or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your doctor, pharmacist, or nurse how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Dupixent
- The active ingredient is dupilumab.
- Each pre-filled syringe contains 300 mg of dupilumab in 2 ml of injectable solution (injectable).
- The other components are L-Arginine monohydrochloride, L-Histidine, L-Histidine monohydrochloride monohydrate, polysorbate 80 (E 433), sodium acetate trihydrate, glacial acetic acid (E 260), sucrose, and water for injectable preparations.
Appearance of the Product and Container Contents
Dupixent is a clear to slightly opalescent solution, colorless to pale yellow, presented in a pre-filled glass syringe with a needle protector.
Dupixent is available as pre-filled syringes of 300 mg in a container containing 1 or 2 pre-filled syringes or in a multiple container containing 6 (3 containers of 2) pre-filled syringes.
Only some pack sizes may be marketed.
Marketing Authorization Holder
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly
France
Manufacturer
SANOFI WINTHROP INDUSTRIE
1051 Boulevard Industriel,
76580 LE TRAIT,
FRANCE
Sanofi-Aventis Deutschland GmbH
Brüningstrasse 50
Industriepark Hoechst
65926 FRANKFURT AM MAIN
GERMANY
Genzyme Ireland Limited
IDA Industrial Park
Old Kilmeaden Road
Waterford
Ireland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 | Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Luxembourg/Luxemburg Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 (Belgique/Belgien) |
Ceská republika Sanofi, s.r.o. Tel: +420 233 086 111 | Magyarország SANOFI-AVENTIS Zrt. Tel.: +36 1 505 0050 |
Danmark Sanofi A/S Tlf: +45 45 16 70 00 | Malta Sanofi S.r.l. Tel: +39 02 39394275 |
Deutschland Sanofi-Aventis Deutschland GmbH Tel.: 0800 04 36 996 Tel. from abroad: +49 69 305 70 13 | Nederland Sanofi B.V. Tel: + 31 20 245 4000 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Norge sanofi-aventis Norge AS Tlf: +47 67 10 71 00 |
Ελλάδα Sanofi-Aventis Μονοπρóσωπη AEBE Τηλ: +30 210 900 16 00 | Österreich sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Polska Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
France Sanofi Winthrop Industrie Tél: 0 800 222 555 Call from abroad: +33 1 57 63 23 23 | Portugal Sanofi - Produtos Farmacêuticos, Lda Tel: +351 21 35 89 400 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | România Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tel: 800 536389 | Suomi/Finland Sanofi Oy Puh/Tel: +358 (0) 201 200 300 |
Κύπρος C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sverige Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50 | United Kingdom(Northern Ireland) sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44 (0) 800 035 2525 |
Date of the Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
Dupixent 300mg solution for injection in pre-filled syringe with needle protector
dupilumab
Instructions for Use
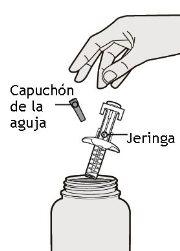
In this drawing, the parts of the Dupixent pre-filled syringe with needle protector are shown.
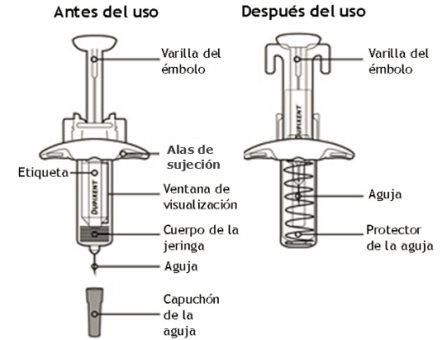
Important Information
This device is a single-use pre-filled syringe. It contains 300 mg of Dupixent for injection under the skin (subcutaneous injection).
Do not attempt to administer the injection to yourself or another person unless you have received training from your healthcare professional. In adolescents from 12 years of age, it is recommended that Dupixent be administered by or under the supervision of an adult. In children under 12 years of age, Dupixent should be administered by a caregiver.
- Read all the instructions carefully before using the syringe.
- Check with your healthcare professional how often the medication should be injected.
- Consult your healthcare professional to show you how to use the syringe correctly before using it for the first time.
- Change the injection site each time you administer the injection.
- Do notuse the syringe if it has been dropped onto a hard surface or if it has been damaged.
- Do notuse the syringe if the needle cap is missing or not properly secured.
- Do nottouch the plunger rod until you are ready for injection.
- Do notinject through clothing.
- Do notattempt to remove air bubbles from the syringe.
- To help prevent accidental needle injuries, each pre-filled syringe comes with a needle protector that automatically activates to cover the needle after administering the injection.
- Neverpull back on the plunger rod.
- Do notreuse the syringe.
Storage of Dupixent
- Keep the syringe(s) out of the reach of children.
- Keep unused syringes in the original container and store them in the refrigerator between 2 °C and 8 °C.
- Do notstore Dupixent at room temperature (< 25 °C) for more than 14 days. If you need to remove the container from the refrigerator permanently, write the date you removed it on the space provided on the outer container, and use Dupixent within the next 14 days.
- Do notshake the syringe at any time.
- Do notheat the syringe.
- Do notfreeze the syringe.
- Do notexpose the syringe to direct sunlight.
Step 1: Remove
Remove the syringe from the container by holding it in the middle of the syringe body.
 Do not remove the needle cap until you are ready for injection.
Do not remove the needle cap until you are ready for injection.
 Do not use the syringe if it has been dropped onto a hard surface or if it has been damaged.
Do not use the syringe if it has been dropped onto a hard surface or if it has been damaged.
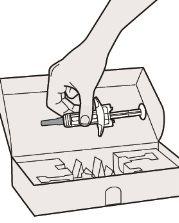
Step 2: Prepare
Make sure you have the following:
- the Dupixent pre-filled syringe
- 1 alcohol swab*
- 1 cotton ball or gauze*
- a sharps container* (see Step 12)
- Items not included in the container
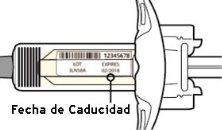
Check the label:
- Check the expiration date.
- Check that you have the correct medication and dose.
 Do not use the syringe if the expiration date has passed.
Do not use the syringe if the expiration date has passed.
 Do not store Dupixent at room temperature for more than 14 days.
Do not store Dupixent at room temperature for more than 14 days.
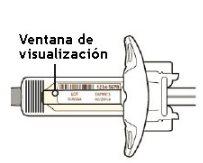
Step 3: Check
Look at the medication through the viewing window of the syringe:
Check that the liquid is clear and colorless to pale yellow.
Note: you may see an air bubble, which is normal.
 Do not use the syringe if the liquid is cloudy or discolored, or if it contains flakes or particles.
Do not use the syringe if the liquid is cloudy or discolored, or if it contains flakes or particles.
Step 4: Wait 45minutes
Place the syringe on a flat surface for at least 45 minutes and let it reach room temperature naturally.
 Do not heat the syringe in a microwave, hot water, or direct sunlight.
Do not heat the syringe in a microwave, hot water, or direct sunlight.
 Do not expose the syringe to direct sunlight.
Do not expose the syringe to direct sunlight.
 Do not store Dupixent at room temperature for more than 14 days.
Do not store Dupixent at room temperature for more than 14 days.
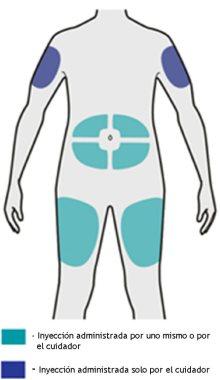
Step 5: Choose
Select the injection site.
- You can inject the medication into your thigh or abdomen, avoiding the area around your navel by about 5 cm.
- If someone else is giving you the injection, it can also be given in the upper arm.
- Change the injection site each time you administer the injection.
 Do not inject into sensitive, damaged, or scarred skin.
Do not inject into sensitive, damaged, or scarred skin.
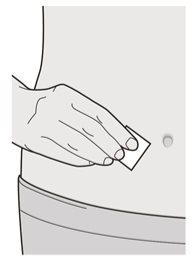
Step 6: Clean
Wash your hands.
Disinfect the injection site with an alcohol swab.
Let the skin dry before proceeding with the injection.
 Do not touch the injection site or blow on it before the injection.
Do not touch the injection site or blow on it before the injection.

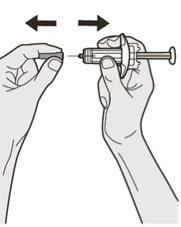
Step 7: Pull
Hold the syringe by the middle of the syringe body, with the needle pointing away from you, and remove the needle cap.
 Do not put the needle cap back on the needle.
Do not put the needle cap back on the needle.
 Do not touch the needle.
Do not touch the needle.
Inject the medication immediately after removing the needle cap.
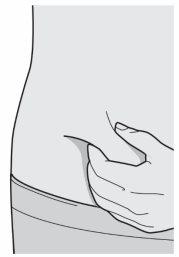
Step 8: Pinch
Pinch a fold of skin at the injection site, as shown in the drawing.
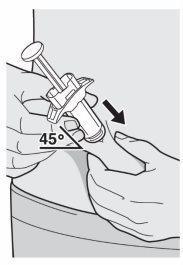
Step 9: Insert
Insert the needle completely into the fold of skin at an angle of approximately 45°.
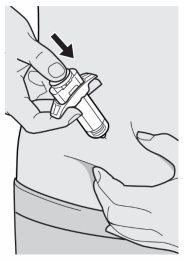
Step 10: Push
Release the pinch.
Push the plunger rod down slowly and continuously until it stops and the syringe is empty.
Note: you will feel a little resistance, which is normal.
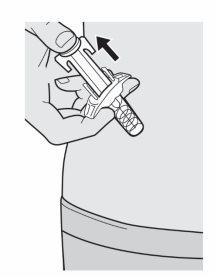
Step 11: Release and Remove
Lift your thumb to release the plunger rod until the needle is covered by the needle protector, then remove the syringe from the injection site.
If you see a little blood, press gently on the injection site with a cotton ball or gauze.
 Do not put the needle cap back on the needle.
Do not put the needle cap back on the needle.
 Do not rub the skin after the injection.
Do not rub the skin after the injection.
Step 12: Dispose
Dispose of the syringe and needle cap in a sharps container.
 Do not put the needle cap back on the needle.
Do not put the needle cap back on the needle.
Always keep the container out of the reach of children.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DUPIXENT 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 200 mgActive substance: dupilumabManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 200 mgActive substance: dupilumabManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 300 mgActive substance: dupilumabManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for DUPIXENT 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about DUPIXENT 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions









