
DEXMEDETOMIDINE KALCEKS 100 micrograms/ml concentrate for infusion solution

How to use DEXMEDETOMIDINE KALCEKS 100 micrograms/ml concentrate for infusion solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
DexmedetomidineKalceks100micrograms/ml concentrate forsolution for infusion EFG
Dexmedetomidine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Dexmedetomidine Kalceks and what is it used for
- What you need to know before you are given Dexmedetomidine Kalceks
- How to use Dexmedetomidine Kalceks
- Possible side effects
- Storing Dexmedetomidine Kalceks
- Contents of the pack and other information
1. What is Dexmedetomidine Kalceks and what is it used for
Dexmedetomidine Kalceks contains an active substance called dexmedetomidine, which belongs to a group of medicines called sedatives. It is used to provide sedation (a state of calm, drowsiness or sleep) in adult patients in intensive care units of hospitals or conscious sedation during various diagnostic or surgical procedures.
2. What you need to know before you are given Dexmedetomidine Kalceks
Dexmedetomidine Kalceks must not be given to you:
- if you are allergic to dexmedetomidine or any of the other ingredients of this medicine (listed in section 6).
- if you have certain heart rhythm disorders (grade 2 or 3 heart block).
- if you have very low blood pressure that does not respond to treatment.
- if you have recently had a stroke or other severe episodes that affect blood supply to the brain.
Warnings and precautions
Before using this medicine, tell your doctor or nurse if you are in any of the following situations, as Dexmedetomidine Kalceks should be used with caution:
- if you have an abnormally slow heart rate (either due to disease or a high level of physical fitness) as it may increase the risk of cardiac arrest
- if you have low blood pressure
- if you have low blood volume, for example after bleeding
- if you have certain heart diseases
- if you are elderly
- if you have a neurological disorder (e.g. head or spinal cord injuries or stroke)
- if you have severe liver problems
- if you have ever developed a severe fever after some medications, especially anesthetics
This medicine may cause a large amount of urine and excessive thirst, contact a doctor if these side effects occur. See section 4 for more information.
Using Dexmedetomidine Kalceks with other medicines
Tell your doctor or nurse if you are using, have recently used or might use any other medicines.
The following medicines may increase the effect of Dexmedetomidine Kalceks:
- medicines that help you sleep or cause sedation (e.g. midazolam, propofol)
- medicines for severe pain (e.g. opioids such as morphine, codeine)
- anesthetic medicines (e.g. sevoflurane, isoflurane)
If you are using medicines that lower your blood pressure and heart rate, taking them with Dexmedetomidine Kalceks may increase this effect. Dexmedetomidine Kalceks should not be used with medicines that can cause temporary paralysis.
Pregnancy and breastfeeding
Dexmedetomidine Kalceks should not be used during pregnancy or breastfeeding, unless clearly necessary. Consult your doctor before using this medicine.
Driving and using machines
Dexmedetomidine Kalceks has a major impact on the ability to drive and use machines. Once you have been given Dexmedetomidine Kalceks, you should not drive, operate machines or work in hazardous situations until the effects have passed completely. Consult your doctor when you can resume these activities and this type of work.
Dexmedetomidine Kalceks contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per ml; this is, essentially “sodium-free”.
3. How to use Dexmedetomidine Kalceks
Intensive care in hospitals
Dexmedetomidine Kalceks is given to you by a doctor or nurse in the intensive care unit of a hospital.
Procedural sedation / conscious sedation
Dexmedetomidine Kalceks is given to you by a doctor or nurse before and/or during diagnostic or surgical procedures that require sedation, i.e. for procedural sedation / conscious sedation.
Your doctor will decide the right dose for you. The amount of Dexmedetomidine Kalceks depends on your age, body weight, general health, the level of sedation needed and how you respond to the medicine. Your doctor may change your dose if necessary and will monitor your heart and blood pressure during treatment.
Dexmedetomidine Kalceks is diluted and given to you as an infusion (drip) in your veins.
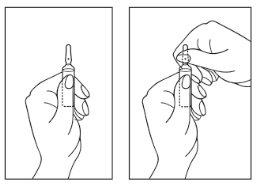
Instructions for opening the ampoule:
- Turn the ampoule with the colored point upwards. If there are any drops of the solution on the top of the ampoule, gently tap with your finger to bring all the solution to the bottom of the ampoule.
- Use both hands to open. While holding the bottom of the ampoule with one hand, use the other hand to break the top of the ampoule in the direction opposite the colored point (see the images below).
After sedation/waking up
- Your doctor will keep you under supervision for a few hours after sedation, to make sure you are well.
- You should not go home unless accompanied.
- Medicines that help you sleep, cause sedation and those intended to relieve severe pain may not be recommended for a period of time after treatment with Dexmedetomidine Kalceks. Consult your doctor about the use of these types of medicines and about the use of alcohol.
If you have been given too much Dexmedetomidine Kalceks
If too much Dexmedetomidine Kalceks has been given to you, your blood pressure may go up or down, your heart beats may be slower, you may breathe more slowly and you may feel more drowsy. Your doctor will know how to treat you based on your condition.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you have any further questions on the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common (affect more than 1 in 10 users)
- slow heart rate
- low or high blood pressure
- change in breathing pattern or breathing stop
Common (affect between 1 and 10 in 100 users)
- chest pain or heart attack
- fast heart rate
- low or high blood sugar levels
- nausea, vomiting or dry mouth
- restlessness
- high temperature
- symptoms after stopping the medicine
Uncommon (affect between 1 and 10 in 1,000 users)
- decreased heart function, cardiac arrest
- stomach swelling
- thirst
- a condition in which there is too much acid in the body
- low albumin level in the blood
- difficulty breathing
- hallucinations
- the medicine is not effective enough
Rare (cannot be estimated from the available data)
- a large amount of urine and excessive thirst – may be symptoms of a hormonal disorder called diabetes insipidus. Contact a doctor if this occurs.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Surveillance System for Human Use: www.notificaRAM.es
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Dexmedetomidine Kalceks
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage conditions.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month shown.
6. Contents of the pack and other information
Composition of Dexmedetomidine Kalceks
- The active substance is dexmedetomidine. Each ml of concentrate contains dexmedetomidine hydrochloride equivalent to 100 micrograms of dexmedetomidine.
- The other ingredients are sodium chloride and water for injections.
Each 2 ml ampoule contains 200 micrograms of dexmedetomidine (as hydrochloride).
Each 4 ml vial contains 400 micrograms of dexmedetomidine (as hydrochloride).
Each 10 ml vial contains 1000 micrograms of dexmedetomidine (as hydrochloride).
The concentration of the final solution after dilution should be 4 micrograms/ml or 8 micrograms/ml.
Appearance of Dexmedetomidine Kalceks and pack contents
Concentrate for solution for infusion (sterile concentrate).
The concentrate is a clear and colorless or yellowish solution.
Dexmedetomidine Kalceks is produced in 2 ml colorless glass ampoules and 4 ml or 10 ml colorless glass vials.
Pack sizes:
5 or 25 ampoules of 2 ml
1 or 4 vials of 4 ml
1 or 4 vials of 10 ml
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
AS KALCEKS
Krustpils iela 71E, Riga, LV‑1057, Latvia
Tel.: +371 67083320
E-mail: [email protected]
You can request more information about this medicine from the local representative of the marketing authorisation holder:
Grindeks Kalceks España, S.L.
c/ José Abascal, 58 2º dcha
28003 Madrid
Spain
This medicine is authorised in the Member States of the European Economic Area under the following names:
Denmark Dexmedetomidin Kalceks
Austria Dexmedetomidin Kalceks 100 Mikrogramm/ml Konzentrat zur Herstellung einer Infusionslösung
Belgium Dexmedetomidine Kalceks 100 microgrammes/ml solution à diluer pour perfusion Dexmedetomidine Kalceks 100 microgram/ml concentraat voor oplossing voor infusie
Dexmedetomidine Kalceks 100 Mikrogramm/ml Konzentrat zur Herstellung einer Infusionslösung
Bulgaria ??????????????? ??????? 100 ??????????/ml ?????????? ?? ?????????? ???????
Croatia Deksmedetomidin Kalceks 100 mikrograma/ml koncentrat za otopinu za infuziju
Czech Republic Dexmedetomidine Kalceks
Estonia Dexmedetomidine Kalceks
Finland Dexmedetomidine Kalceks
France DEXMEDETOMIDINE KALCEKS 100 microgrammes/mL, solution à diluer pour perfusion
Germany Dexmedetomidin Ethypharm 100 Mikrogramm/ml Konzentrat zur Herstellung einer Infusionslösung
Hungary Dexmedetomidine Kalceks 100 mikrogramm/ml koncentrátum oldatos infúzióhoz
Ireland Dexmedetomidine 100 micrograms/ml concentrate for solution for infusion
Italy Dexmedetomidina Kalceks
Latvia Dexmedetomidine Kalceks 100 mikrogrami/ml koncentrats infuziju škiduma pagatavošanai
Lithuania Dexmedetomidine Kalceks 100 mikrogramu/ml koncentratas infuziniam tirpalui
Norway Dexmedetomidine Kalceks
Poland Dexmedetomidine Kalceks
Portugal Dexmedetomidina Kalceks
Romania Dexmedetomidina Kalceks 100 micrograme/ml concentrat pentru solutie perfuzabila
Slovakia Dexmedetomidine Kalceks 100 mikrogramov/ml infúzny koncentrát
Slovenia Deksmedetomidin Kalceks 100 mikrogramov/ml koncentrat za raztopino za infundiranje
Spain Dexmedetomidina Kalceks 100 microgramos/ml concentrado para solución para perfusión EFG
Sweden Dexmedetomidine Kalceks
The Netherlands Dexmedetomidine Kalceks 100 microgram/ml concentraat voor oplossing voor infusie
Date of last revision of this leaflet:September 2023.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
DexmedetomidineKalceks100micrograms/ml concentrate forsolution for infusion EFG
Route of administration
Dexmedetomidine Kalceks should be administered by healthcare professionals experienced in the management of patients who require intensive care or in the management of anesthesia in patients in the operating room. It should be administered only as a diluted intravenous infusion using a controlled infusion device.
Preparation of the solution
Dexmedetomidine Kalceks can be diluted in glucose 50 mg/ml (5%), Ringer's solution, lactated Ringer's solution, mannitol or sodium chloride 9 mg/ml (0.9%) injectable solution to achieve the required concentration of 4 micrograms/ml or 8 micrograms/ml before administration. See the table below for the volumes needed to prepare the infusion.
In case a concentration of 4 micrograms/ml is required:
Volume of Dexmedetomidine Kalceks 100 micrograms/ml concentrate for solution for infusion EFG | Volume of diluent | Total volume of infusion |
2 ml | 48 ml | 50 ml |
4 ml | 96 ml | 100 ml |
10 ml | 240 ml | 250 ml |
20 ml | 480 ml | 500 ml |
In case a concentration of 8 micrograms/ml is required:
Volume of Dexmedetomidine Kalceks 100 micrograms/ml concentrate for solution for infusion EFG | Volume of diluent | Total volume of infusion |
4 ml | 46 ml | 50 ml |
8 ml | 92 ml | 100 ml |
20 ml | 230 ml | 250 ml |
40 ml | 460 ml | 500 ml |
The solution should be gently shaken to mix well.
This medicine should be visually inspected for particles and coloration before administration.
This medicine has been shown to be compatible when administered with the following intravenous fluids and medicines:
Lactated Ringer's solution, glucose 5% solution, sodium chloride 0.9% injectable solution, mannitol 20% solution, thiopental sodium, etomidate, vecuronium bromide, pancuronium bromide, succinylcholine, atracurium besylate, mivacurium chloride, rocuronium bromide, glycopyrrolate, phenylephrine HCl, atropine sulfate, dopamine, noradrenaline, dobutamine, midazolam, morphine sulfate, fentanyl citrate, and a plasma substitute.
Incompatibilities
Compatibility studies have shown potential for adsorption of dexmedetomidine to some types of natural rubber. Although dexmedetomidine is dosed to effect, it is recommended to use components with synthetic or coated natural rubber seals.
Shelf life after dilution
The chemical and physical stability of the diluted infusion has been demonstrated for 36 hours at 25°C and under refrigerated conditions (2°C – 8°C).
From a microbiological point of view, the product should be used immediately. If not used immediately, the storage times and conditions prior to use are the responsibility of the user and normally should not be longer than 24 hours between 2° and 8°C, unless the dilution has been made in controlled and validated aseptic conditions.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DEXMEDETOMIDINE KALCEKS 100 micrograms/ml concentrate for infusion solutionDosage form: INJECTABLE PERFUSION, 100 micrograms dexmedetomidine/mlActive substance: dexmedetomidineManufacturer: Orion CorporationPrescription requiredDosage form: INJECTABLE PERFUSION, 100 µg/mlActive substance: dexmedetomidineManufacturer: Orion CorporationPrescription requiredDosage form: INJECTABLE PERFUSION, 100 micrograms dexmedetomidine/mlActive substance: dexmedetomidineManufacturer: Orion CorporationPrescription required
Online doctors for DEXMEDETOMIDINE KALCEKS 100 micrograms/ml concentrate for infusion solution
Discuss questions about DEXMEDETOMIDINE KALCEKS 100 micrograms/ml concentrate for infusion solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















