
DARUNAVIR TARBIS 400 mg FILM-COATED TABLETS

How to use DARUNAVIR TARBIS 400 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Darunavir Tarbis 400 mg film-coated tablets EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Darunavir Tarbis and what is it used for
- What you need to know before you take Darunavir Tarbis
- How to take Darunavir Tarbis
- Possible side effects
- Storage of Darunavir Tarbis
- Contents of the pack and other information
1. What is Darunavir Tarbis and what is it used for
What is Darunavir Tarbis?
Darunavir Tarbis contains the active substance darunavir. Darunavir Tarbis is an antiretroviral medicine used in the treatment of infection by the Human Immunodeficiency Virus (HIV). It belongs to a group of medicines called protease inhibitors. Darunavir Tarbis reduces the amount of HIV in your body. This will improve your immune system and reduce the risk of diseases associated with HIV infection.
What is it used for?
The 400-milligram tablet of Darunavir Tarbis is used to treat adults and children (from 3 years of age, weighing at least 40 kilograms) infected with HIV and
- who have not used antiretroviral medicines before.
- in certain patients who have used antiretroviral medicines before (this will be determined by your doctor).
This medicine must be taken with a low dose of cobicistat or ritonavir and other HIV medicines. Your doctor will explain the combination of medicines that is best for you.
2. What you need to know before you take Darunavir Tarbis
Do not take Darunavir Tarbis
- if you are allergicto darunavir or any of the other ingredients of this medicine (listed in section 6) or to cobicistat or ritonavir.
- if you have severe liver problems. Ask your doctor if you are not sure about the severity of your liver disease. You may need to have some additional tests.
Do not combine Darunavir Tarbis with any of the following medicines
If you are taking any of these medicines, consult your doctor to change to another medicine.
Medicine | Purpose of the medicine |
Avanafil | treatment of erectile dysfunction |
Astemizoleor terfenadine | treatment of allergy symptoms |
Triazolamand midazolam(by mouth) | to help you sleep and/or relieve anxiety |
Cisapride | treatment of stomach problems |
Colchicine(if you have kidney and/or liver problems) | treatment of gout or familial Mediterranean fever |
Lurasidone, pimozide, quetiapine, or sertindole | treatment of psychiatric problems |
Alkaloids of the ergot of the ryesuch as ergotamine, dihydroergotamine, ergometrineand methylergonovine | treatment of migraine-type headaches |
Amiodarone, bepridil, dronedarone, ivabradine, quinidine, ranolazine | treatment of certain heart rhythm disorders, for example, irregular heartbeats |
Lovastatin, simvastatin, and lomitapide | to lower cholesterol levels |
Rifampicin | treatment of certain infections such as tuberculosis |
The combination of medicines lopinavir/ritonavir | this HIV medicine belongs to the same class as Darunavir Tarbis 400 mg film-coated tablets EFG |
Elbasvir/grazoprevir | to treat hepatitis C infection |
Alfuzosin | treatment of enlarged prostate |
Sildenafil | treatment of high blood pressure in the pulmonary circulation |
Dabigatran, ticagrelor | to help stop platelet aggregation during treatment of patients with a history of heart attack |
Naloxegol | to treat opioid-induced constipation |
Dapoxetine | to treat premature ejaculation |
Domperidone | to treat nausea and vomiting |
Do not combine this medicine with products containing St. John's Wort (Hypericum perforatum).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to take Darunavir Tarbis.
Darunavir does not cure HIV infection. While you are taking this medicine, you can still transmit HIV to others, although effective antiviral treatment reduces the risk. Consult your doctor about the precautions needed to avoid infecting other people.
People taking darunavir may develop other infections or diseases associated with HIV infection. You must keep in regular contact with your doctor.
People taking darunavir may develop a skin rash. It is not common for the rash to be severe or life-threatening. Please consult your doctor if you develop a rash.
Patients taking darunavir and raltegravir (for HIV infection) may develop rashes (usually mild or moderate) more frequently than patients taking either of the two medicines separately.
Tell your doctor about your situation BEFORE and DURING your treatment
Make sure you check the following points and inform your doctor if any apply to you.
- Tell your doctor if you have had any liver disease, including hepatitis B or C infection. Your doctor will assess the severity of your liver disease before deciding if you can take this medicine.
- Tell your doctor if you have diabetes. Darunavir may cause an increase in blood sugar levels.
- Tell your doctor immediately if you notice any symptoms of infection(for example, swollen lymph nodes and fever). In some patients with advanced HIV infection and a history of opportunistic infections, signs and symptoms of inflammation due to previous infections may appear soon after starting HIV treatment. It is believed that these symptoms are due to an improvement in the body's immune response, which enables it to fight off infections that were present without any apparent symptoms.
- In addition to opportunistic infections, autoimmune disorders (a condition that occurs when the immune system attacks healthy body tissue) may also appear after you have started taking medicines for the treatment of your HIV infection. Autoimmune disorders may appear many months after starting treatment. If you notice any symptoms of infection or other symptoms such as muscle weakness, weakness starting in the hands and feet and rising towards the trunk of the body, palpitations, tremors, or hyperactivity, inform your doctor immediately to receive the necessary treatment.
- Tell your doctor if you have hemophilia. Darunavir may increase the risk of bleeding.
- Tell your doctor if you are allergic to sulfonamides(for example, used to treat certain infections).
- Tell your doctor if you notice any bone or muscle problems.Some patients using combination antiretroviral therapy may develop a bone disease called osteonecrosis (death of bone tissue caused by lack of blood supply to the bone). Some of the many risk factors for developing this disease include the duration of combination antiretroviral therapy, the use of corticosteroids, alcohol consumption, severe immunodepression, and a higher body mass index. The signs of osteonecrosis are pain, discomfort, and stiffness of the joints (especially the hip, knee, and shoulder) and difficulty moving. If you notice any of these symptoms, please consult your doctor.
Elderly population
Darunavir has only been used in a limited number of patients aged 65 years or older. If you belong to this age group, please talk to your doctor to see if you can use this medicine.
Children and adolescents
The 400-milligram tablet of Darunavir Tarbis must not be used in children under 3 years of age or weighing less than 40 kilograms.
Taking Darunavir Tarbis with other medicines
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines.
Some medicines must not be combinedwith this medicine. The list can be found in the section "Do not combine Darunavir Tarbis with any of the following medicines:"
In most cases, darunavir can be combined with HIV medicines that belong to other classes [e.g., NRTIs (nucleoside reverse transcriptase inhibitors), NNRTIs (non-nucleoside reverse transcriptase inhibitors), CCR5 antagonists, and FIs (fusion inhibitors)]. Darunavir has not been tested with cobicistat or ritonavir with all protease inhibitors (PIs) and must not be used with other HIV protease inhibitors. In some cases, it may be necessary to change the dose of the other medicines. Therefore, if you are taking other anti-HIV medicines, always inform your doctor and carefully follow their instructions on which medicines can be combined.
The following products may reduce the effectiveness of darunavir. Inform your doctor if you are taking:
- Phenobarbital, phenytoin(to prevent seizures)
- Dexamethasone(corticosteroid)
- Efavirenz(for HIV infection)
- Boceprevir(to treat hepatitis C infection)
- Rifapentine, rifabutin(medicines to treat certain infections such as tuberculosis)
- Saquinavir(for HIV infection).
Darunavir may also affect the effects of other medicines. Inform your doctor if you are taking:
- Amlodipine, diltiazem, disopyramide, carvedilol, felodipine, flecainide, lidocaine, metoprolol, mexiletine, nifedipine, nicardipine, propafenone, timolol, verapamil(for heart disorders) because the therapeutic or adverse effects of these medicines may be increased.
- Apixaban, edoxaban, rivaroxaban, warfarin(to reduce blood clotting) because the therapeutic or adverse effects of these medicines may be altered; your doctor may perform blood tests.
- Hormonal contraceptives based on estrogens and hormone replacement therapy. Darunavir may reduce their effectiveness. For birth control, alternative non-hormonal contraceptive methods are recommended.
- Ethinylestradiol/drospirenone. Darunavir may increase the risk of elevated potassium levels due to the effect of drospirenone.
- Atorvastatin, pravastatin, rosuvastatin(to lower blood cholesterol levels). There may be a higher risk of muscle damage. Your doctor will determine which cholesterol-lowering treatment is best for you based on your personal circumstances.
- Clarithromycin(antibiotic)
- Cyclosporine, everolimus, tacrolimus, sirolimus(to suppress the immune system) because the therapeutic or adverse effects of these medicines may be increased. Your doctor may perform additional tests.
- Corticosteroids, including betamethasone, budesonide, fluticasone, mometasone, prednisone, triamcinolone.These medicines are used to treat allergies, asthma, inflammatory bowel diseases, inflammatory eye, joint, and muscle diseases, and other inflammatory conditions. If alternatives cannot be used, their use should only be done after a clinical evaluation and with close monitoring by your doctor to assess the adverse effects of corticosteroids.
- Buprenorphine/naloxone(medicines for the treatment of opioid dependence)
- Salmeterol(medicine for the treatment of asthma)
- Artemether/lumefantrine(a combination of medicines to treat malaria)
- Dasatinib, everolimus, irinotecan, nilotinib, vinblastine, vincristine(to treat cancer)
- Sildenafil, tadalafil, vardenafil(for erectile dysfunction or to treat a heart and lung disorder called pulmonary arterial hypertension)
- Glecaprevir/pibrentasvir, simeprevir(to treat hepatitis C infection)
- Fentanyl, oxycodone, tramadol(to treat pain)
- Fesoterodine, solifenacin(to treat urological disorders).
In certain cases, it will be necessary to modify the dose of some medicines since their combination may affect the therapeutic or adverse effects of these or of darunavir.
Tell your doctor if you are taking:
- Alfentanil(injectable analgesic with strong and short action used in surgical procedures)
- Digoxin(for the treatment of certain heart disorders)
- Clarithromycin(antibiotic)
- Itraconazole, isavuconazole, fluconazole, posaconazole, clotrimazole(to treat fungal infections). Voriconazole can only be administered after a medical evaluation.
- Rifabutin(against bacterial infections)
- Sildenafil, vardenafil, tadalafil(for erectile dysfunction or high blood pressure in the pulmonary circulation)
- Amitriptyline, desipramine, imipramine, nortriptyline, paroxetine, sertraline, trazodone(to treat depression and anxiety)
- Maraviroc(to treat HIV infection)
- Methadone(to treat narcotic dependence)
- Carbamazepine, clonazepam(to prevent epileptic seizures or to treat certain types of neuropathic pain)
- Colchicine(for the treatment of gout or familial Mediterranean fever)
- Bosentan(for the treatment of high blood pressure in the pulmonary circulation)
- Buspirone, chlordiazepoxide, diazepam, estazolam, flurazepam, midazolam when administered by injection, zolpidem(sedative agents)
- Perphenazine, risperidone, thioridazine(to treat psychiatric conditions)
- Metformin(to treat type 2 diabetes).
This is nota complete list of medicines. Inform your doctor about allthe medicines you are taking.
Taking Darunavir Tarbis with food and drinks
See section 3 "How to take Darunavir Tarbis"
Pregnancy and breastfeeding
Tell your doctor immediately if you are pregnant, plan to become pregnant, or are breastfeeding. Pregnant or breastfeeding women must not take darunavir with ritonavir unless their doctor specifically instructs them to do so. Pregnant or breastfeeding women must not take darunavir with cobicistat.
It is recommended that women infected with HIV do not breastfeed their babies because there is a possibility that the babies may become infected with HIV through breast milk, as well as the unknown effects of the medicine on the babies.
Driving and using machines
Do not operate tools or machines or drive if you experience dizziness after taking this medicine.
3. How to take Darunavir Tarbis
Follow the administration instructions for this medication contained in this prospectus or as indicated by your doctor, pharmacist, or nurse. In case of doubt, ask your doctor, pharmacist, or nurse. Do not stop taking darunavir or cobicistat or ritonavir without consulting your doctor first, even if you feel better.
After starting treatment, do not change the dose or form of the dose or interrupt treatment without your doctor's instructions.
The 400-milligram Darunavir Tarbis tablets are used only to obtain the 800-milligram once-daily dosage.
Dose for adults who have not taken antiretroviral medications before (will be determined by your doctor)
The normal dose of darunavir is 800 milligrams (2 tablets of 400 milligrams of darunavir or 1 tablet containing 800 milligrams of darunavir) once a day.
You should take darunavir every day and always in combination with 150 milligrams of cobicistat or 100 milligrams of ritonavir and with food. Darunavir does not work properly without cobicistat or ritonavir and food. Before taking the darunavir and cobicistat or ritonavir tablets, you should eat 30 minutes before. The type of food is not important. Do not interrupt treatment with darunavir or cobicistat or ritonavir without consulting your doctor first, even if you feel better.
Instructions for adults
- Take two 400-milligram tablets at the same time, once a day, every day.
- Always take darunavir with 150 milligrams of cobicistat or 100 milligrams of ritonavir.
- Take the tablets with food.
- Swallow the tablets with a drink, which can be water or milk.
- Take the other HIV medications used in combination with darunavir and cobicistat or ritonavir as your doctor recommends.
- The 100-milligram-per-milliliter oral suspension of darunavir has been developed for use in children, but in some cases, it can also be used in adults.
Dose for adults who have taken antiretroviral medications before (will be determined by your doctor)
The dose is:
- 800 milligrams of darunavir (2 tablets containing 400 milligrams of darunavir or 1 tablet containing 800 milligrams of darunavir) along with 150 milligrams of cobicistat or 100 milligrams of ritonavir once a day.
Or
- 600 milligrams of darunavir (1 tablet containing 600 milligrams of darunavir) along with 100 milligrams of ritonavir twice a day.
Please talk to your doctor about which dose is correct for you.
Dose for children from 3 years of age, weighing more than 40 kilograms, who have not taken antiretroviral medications before (will be determined by your child's doctor)
- The usual dose of darunavir is 800 milligrams (2 tablets containing 400 milligrams of darunavir or 1 tablet containing 800 milligrams of darunavir) along with 100 milligrams of ritonavir once a day.
Dose for children from 3 years of age, weighing more than 40 kilograms, who have taken antiretroviral medications before (will be determined by your child's doctor)
The dose is:
- 800 milligrams of darunavir (2 tablets containing 400 milligrams of darunavir or 1 tablet containing 800 milligrams of darunavir) along with 100 milligrams of ritonavir once a day.
Or
- 600 milligrams of darunavir (1 tablet containing 600 milligrams of darunavir) along with 100 milligrams of ritonavir twice a day.
Please talk to your doctor about which dose is correct for you.
Instructions for children from 3 years of age, weighing more than 40 kilograms
- Take 800 milligrams of darunavir (2 tablets containing 400 milligrams of darunavir or 1 tablet containing 800 milligrams of darunavir) at the same time once a day, every day.
- Always take darunavir with 100 milligrams of ritonavir.
- Take the tablets with food.
- Swallow the tablets with a liquid, such as water or milk.
- Take the other medications used in combination with darunavir and ritonavir as your doctor recommends.
Child-resistant cap removal
The plastic bottle has a child-resistant safety cap and is opened as follows:
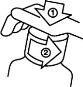 Push the plastic cap down, turning it counterclockwise at the same time.
Push the plastic cap down, turning it counterclockwise at the same time.- Remove the cap by unscrewing.
If you take more Darunavir Tarbis than you should
Inform your doctor, pharmacist, or nurse immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take Darunavir Tarbis
If you realize within 12 hours, take the tablets immediately. Always take the dose with cobicistat or ritonavir and with food. If you realize after 12 hours, skip that dose and make the next one as usual. Do not take a double dose to make up for missed doses.
Do not stop taking Darunavir Tarbis without talking to your doctor first
HIV medications can make you feel better. Even if you feel better, do not stop taking this medication. Consult your doctor first.
If you have any other questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
During HIV treatment, there may be an increase in weight and glucose and lipid levels in the blood. This may be partly related to the recovery of health and lifestyle, and in the case of blood lipids, sometimes to HIV medications themselves. Your doctor will monitor these changes.
Like all medications, this medication can cause side effects, although not all people experience them.
Tell your doctor if you develop any of the following side effects.
There have been reports of liver problems that can occasionally be severe. Your doctor will perform a blood test before you start treatment with darunavir. If you have a chronic infection caused by hepatitis B or C, your doctor will frequently check your blood tests, as there is a higher likelihood of developing liver problems. Talk to your doctor about the signs and symptoms of liver problems. These may include yellowing of the skin and the whites of the eyes, darkening (tea-colored) urine, pale stools, nausea, vomiting, loss of appetite, or pain, discomfort, or tenderness on the right side below your ribs.
Skin rash (more frequent when used in combination with raltegravir), itching. The skin rash is usually mild to moderate. A skin rash can also be a symptom of a rare and serious situation. Therefore, it is essential to talk to your doctor if you have a rash. Your doctor will advise you on how to control the symptoms or if you should stop taking darunavir.
Other serious side effects were diabetes (frequent) and pancreatitis (uncommon). Very common side effects(may affect more than 1 in 10 patients)
- diarrhea.
Common side effects(may affect up to 1 in 10 patients)
- vomiting, nausea, abdominal pain or distension, upper abdominal pain (dyspepsia), flatulence
- headache, fatigue, dizziness, drowsiness, numbness, tingling, or pain in the hands or feet, weakness, difficulty staying asleep.
Uncommon side effects(may affect up to 1 in 100 patients)
- chest pain, changes in the electrocardiogram, rapid heart rate
- decreased or abnormal skin sensitivity, tingling, attention disorder, memory loss, difficulty maintaining balance
- breathing difficulties, cough, nasal bleeding, throat irritation
- stomach or mouth inflammation, heartburn, nausea, dry mouth, abdominal discomfort, constipation, belching
- kidney failure, kidney stones, difficulty urinating, excessive or frequent urination, sometimes at night
- hives, severe swelling of the skin and other tissues (especially the lips or eyes), eczema, excessive sweating, night sweats, hair loss, acne, scaly skin, nail discoloration
- muscle pain, muscle sensitivity or weakness, muscle cramps or weakness, pain in the limbs, osteoporosis
- reduced thyroid gland function. This can be seen in a blood test.
- hypertension (high blood pressure), flushing
- red or dry eyes
- fever, swelling of the lower limbs due to fluid retention, discomfort, irritability, pain
- infection symptoms, simple herpes
- erectile dysfunction, breast enlargement
- sleep disorders, drowsiness, depression, anxiety, abnormal dreams, decreased sexual desire.
Rare side effects(may affect up to 1 in 1,000 patients)
- a reaction called DRESS (severe rash, which may be accompanied by fever, fatigue, swelling of the face or lymph nodes, increased eosinophils, liver, kidney, or lung damage)
- myocardial infarction, slow heart rate, palpitations
- visual impairment
- chills, strange sensation
- a feeling of confusion or disorientation, altered mood, agitation
- fainting, epileptic seizure, changes or loss of taste
- mouth ulcers, vomiting blood, lip inflammation, dry lips, tongue with thrush
- nasal discharge
- skin lesions, dry skin
- muscle stiffness or joint pain, joint pain with or without inflammation
- changes in some blood cell or biochemical values. These changes can be seen in blood and/or urine tests. Your doctor will explain them to you. For example: increased white blood cells.
Some side effects are typical of HIV medications that belong to the same family as darunavir. These are:
- muscle pain, sensitivity, or weakness. In rare cases, these muscle disorders can be severe.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this prospectus.
You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Darunavir Tarbis
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the box and bottle, after CAD. The expiration date refers to the last day of the month indicated.
This medication does not require special storage conditions.
Medications should not be thrown down the drain or into the trash. Deposit the packaging and medications you no longer need at the SIGRE Point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Darunavir Tarbis 400 mg film-coated tablets EFG
- The active ingredient is darunavir. Each tablet contains 400 milligrams of darunavir.
- The other ingredients are anhydrous colloidal silica (E551), silicified microcrystalline cellulose (E460), crospovidone (E1202), and magnesium stearate (E470b). The tablet coating contains partially hydrolyzed polyvinyl alcohol, macrogol, titanium dioxide (E171), talc (E553b), and yellow iron oxide (E172).
Appearance of Darunavir Tarbis 400 mg film-coated tablets EFG and package contents
Oval, biconvex, film-coated yellow tablets, approximately 15.7 mm in length and 7.9 mm in width, engraved with a 'V' on one side and a '4' on the other.
Blister pack:
Aluminum blisters in a cardboard box. Each box contains single-dose film-coated tablets in blisters of 30 x 1, 60 x 1, or 90 x 1 units.
Plastic bottle:
High-density polyethylene (HDPE) white opaque plastic bottle in a cardboard box. The bottle contains a desiccant gel silica container. This container should be left inside the bottle to protect the tablets and should not be swallowed.
Each box contains 60 film-coated tablets.
Darunavir Tarbis is also available in 600 mg and 800 mg film-coated tablets.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Tarbis Farma S.L.
Gran Vía Carlos III, 94
08028 Barcelona
Spain
Manufacturer
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park
PLA 3000 Paola
Malta
Amarox Pharma B.V.
Rouboslaan 32
2252 TR Voorschoten
Netherlands
This medication is authorized in EEA Member States under the following names:
Netherlands: Darunavir Amarox 400 mg film-coated tablets
Sweden: Darunavir Amarox 400 mg film-coated tablets
Germany: Darunavir Amarox 400 mg film-coated tablets
Spain: Darunavir Tarbis 400 mg film-coated tablets EFG
Date of the last revision of this prospectus:March 2020
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DARUNAVIR TARBIS 400 mg FILM-COATED TABLETSDosage form: TABLET, 600 mgActive substance: darunavirManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 800 mgActive substance: darunavirManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 400 mgActive substance: darunavirManufacturer: Krka D.D. Novo MestoPrescription required
Online doctors for DARUNAVIR TARBIS 400 mg FILM-COATED TABLETS
Discuss questions about DARUNAVIR TARBIS 400 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















