CUVITRU 200 MG/ML SOLUTION FOR SUBCUTANEOUS INJECTION

How to use CUVITRU 200 MG/ML SOLUTION FOR SUBCUTANEOUS INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cuvitru 200mg/ml, solution for subcutaneous injection
Human normal immunoglobulin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Cuvitru is and what it is used for
- What you need to know before you use Cuvitru
- How to use Cuvitru
- Possible side effects
- Storage of Cuvitru
- Contents of the pack and other information
1. What Cuvitru is and what it is used for
What Cuvitru is
Cuvitru belongs to a group of medicines called "human normal immunoglobulins". Immunoglobulins are also known as antibodies and are found in the blood of healthy people. Antibodies are part of the immune system (the body's natural defenses) and help the body to fight infections.
How Cuvitru works
Cuvitru is made from the blood of healthy people. The medicine works in the same way as the immunoglobulins that are naturally present in the blood.
What Cuvitru is used for
Cuvitru is used in patients with a weak immune system who do not have enough antibodies in their blood and tend to get frequent infections. Regular administration of Cuvitru can increase the abnormally low levels of immunoglobulins in the blood to normal levels (replacement therapy).
Cuvitru is prescribed for:
- patients with a congenital lack of antibody production (primary immunodeficiency syndromes);
- patients who suffer from severe or recurrent infections due to a weakened immune system resulting from other diseases or treatments (secondary immunodeficiency syndromes).
2. What you need to know before you use Cuvitru
Do not use Cuvitru:
- if you are allergic to immunoglobulins or any of the other ingredients of this medicine (listed in section 6)
- if you have antibodies against immunoglobulin A (IgA) in your blood. This may occur if you have an IgA deficiency. As Cuvitru contains traces of IgA, you may have an allergic reaction
- in a blood vessel (by intravenous route) or in a muscle (by intramuscular route).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Cuvitru.
If Cuvitru remains in siliconized syringes for more than two hours, visible particles may form. Follow strictly the detailed instructions given in the "Method and route of administration" section of this leaflet.
Allergic reactions
You may be allergic to immunoglobulins and not know it. Allergic reactions, such as a sudden drop in blood pressure or anaphylactic shock (a sudden drop in blood pressure along with other symptoms such as swelling of the throat, difficulty breathing, and skin rash), are rare but may occur occasionally even if you have not had problems before with similar treatments. You are at greater risk of having an allergic reaction if you have an IgA deficiency with anti-IgA antibodies. Talk to your doctor or nurse before treatment if you have an IgA deficiency. Cuvitru contains residual amounts of IgA, which may increase the risk of an allergic reaction. The signs or symptoms of these rare allergic reactions include:
- feeling of dizziness, drowsiness, or loss of consciousness;
- skin rash and itching, swelling of the mouth or throat, difficulty breathing, wheezing;
- abnormal heart rate, chest pain, bluish discoloration of the lips or fingers and toes;
- blurred vision.
Your doctor or nurse will infuse Cuvitru slowly and carefully at first and will monitor you closely during the first infusions so that if an allergic reaction occurs, it can be detected and treated immediately.
- If you notice any of these signs during the infusion, tell your doctor or nurse immediately. They will decide whether to reduce the infusion rate or stop it completely.
Monitoring during infusion
Certain side effects may occur more frequently if:
- you are receiving Cuvitru for the first time;
- you have received another immunoglobulin and have switched to Cuvitru;
- it has been a long time since you last received Cuvitru.
- In such cases, you will be closely monitored during the first infusion and for the first hour after the infusion has finished.
In other cases, you will be closely monitored during the infusion and for at least 20 minutes after you have received Cuvitru.
Special patient groups
Your doctor will take special precautions if you are overweight, elderly, diabetic, have high blood pressure, low blood volume (hypovolemia), or vascular problems (vascular diseases). In these cases, immunoglobulins may increase the risk of heart attack, stroke, pulmonary embolism, or deep vein thrombosis, although this only occurs in very rare cases.
Your doctor will also take special precautions if you have or have had kidney problems or if you are taking medicines that may harm your kidneys (nephrotoxic medicines), as there is a very small possibility of acute kidney failure.
Inflammation of the membranes covering the brain (aseptic meningitis)
Infusions of products containing immunoglobulins, including Cuvitru, may cause inflammation of the membranes covering the brain. Stopping treatment with immunoglobulin may lead to a reduction in aseptic meningitis for several days. The syndrome usually starts from several hours to 2 days after treatment with immunoglobulin.
Talk to your doctor if you experience the following signs and symptoms: severe headache, stiff neck, drowsiness, fever, nausea, vomiting, sensitivity to light, and discomfort caused by light, after receiving Cuvitru. Your doctor will decide whether further tests are needed and whether Cuvitru administration should be continued.
Destruction of red blood cells (hemolysis)
Cuvitru contains antibodies from blood groups that may cause destruction of red blood cells and hemolytic anemia.
Effects on blood tests
Cuvitru contains many different antibodies, some of which may affect blood tests (serological tests).
- Before you have a blood test, tell your doctor about your treatment with Cuvitru.
Home treatment
You and/or your caregiver will be trained to detect the first signs of side effects, especially allergic reactions. During the infusion, you or your caregiver should watch for the first signs of side effects (for more details, see section 4, "Possible side effects").
- If you experience any side effect, you or your caregiver should stop the infusion immediately and contact a doctor.
- If you experience a severe side effect, you or your caregiver should seek immediate emergency treatment.
Information about the source material of Cuvitru
Cuvitru is produced from human plasma (the liquid part of the blood). When medicines are made from blood or plasma, certain measures are taken to prevent the transmission of infections to patients.
These include:
- careful selection of blood and plasma donors to ensure the exclusion of donors who are at risk of carrying infectious diseases;
- testing of each donation and plasma pool for viruses or infections;
- inclusion of steps in the processing of blood and plasma to inactivate or remove viruses.
Despite these measures, when medicines derived from blood or plasma are administered, the possibility of transmitting infectious agents cannot be completely ruled out. This also applies to emerging or unknown viruses and infectious agents.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and for non-enveloped viruses such as hepatitis A virus and parvovirus B19.
Immunoglobulins have not been associated with hepatitis A or parvovirus B19 infections, probably because the antibodies associated with these infections (and which are present in Cuvitru) provide protection.
It is strongly recommended that each time you receive a dose of Cuvitru, the following information is recorded in the treatment diary:
- the date of administration;
- the batch number of the medicine, and
- the volume injected, the rate of administration, the number, and the sites of infusion.
Children and adolescents
The warnings and precautions included apply to both adults and children.
Using Cuvitru with other medicines
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
Vaccinations
Cuvitru may reduce the effect of some vaccines, such as measles, rubella, mumps, and varicella (live virus vaccines). Therefore, after receiving Cuvitru, you may need to wait up to 3 months before receiving certain vaccines. You may need to wait up to 1 year after receiving the last dose of Cuvitru before you can receive the measles vaccine.
- Before you are vaccinated, tell your doctor or nurse about your treatment with Cuvitru.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
No clinical trials have been conducted with Cuvitru in pregnant or breastfeeding women. However, experience with immunoglobulins suggests that no harmful effects are expected during pregnancy or in the newborn.
If you are breastfeeding and receive Cuvitru, the antibodies in the medicine may also be found in breast milk and may protect the child from certain infections.
Experience with immunoglobulins suggests that no harmful effects on fertility are expected.
Driving and using machines
During treatment with Cuvitru, patients may experience side effects (e.g., dizziness or nausea) that may affect their ability to drive and use machines. If this occurs, wait until the reactions have disappeared.
3. How to use Cuvitru
Follow your doctor's administration instructions for this medication exactly. If in doubt, consult your doctor again.
Cuvitru must be infused under the skin (subcutaneous or SC administration).
Your doctor or nurse will start the treatment with Cuvitru, but once you have received the first infusions under medical supervision and you (and/or your caregiver) are properly trained, you can use the medication at home. You and your doctor will decide if you can use Cuvitru (e.g., by using an infusion pump or manual administration with a syringe) at home. Do not start treatment with Cuvitru at home until you have received complete instructions.
Dosage
Your doctor will calculate the correct dose based on your body weight, previous treatments you have received, and your response to treatment.
Your doctor will decide if you need a loading dose (for adults or children) of at least 1.0 to 2.5 ml/kg of body weight, divided over several days. After this, you will receive Cuvitru regularly, from once a day to once every two weeks; the cumulative monthly dose will be approximately 1.5 to 5 ml/kg of body weight (for primary immunodeficiency syndromes) or approximately 1.0 to 2.0 ml/kg of body weight (for secondary immunodeficiency syndromes). Your doctor may adjust the dose depending on your response to treatment.
Do not change the dose or dosing interval without consulting your doctor. If you think you should receive Cuvitru more or less frequently, consult your doctor. If you think you have missed a dose, consult your doctor as soon as possible.
Starting treatment
Treatment will be started by a doctor or nurse with experience in treating patients with a weak immune system and in training patients for home treatment. You will be closely monitored during the infusion and for at least 1 hour after the infusion to see if you tolerate the medication well. Initially, your doctor or nurse will use a slow infusion rate and gradually increase it during the first infusion and subsequent ones. Once the doctor or nurse has found the right dose and infusion rate for you, you can administer the treatment at home.
Home treatment
Cuvitru can be administered by yourself or your caregiver. Both of you will be trained by a doctor or nurse with experience in handling and treating patients like you. The doctor or nurse will be with you during the first treatments.
You will be trained in:
- germ-free (aseptic) infusion techniques;
- the use of an administration device (if necessary);
- maintaining a treatment diary, and
- the measures to be taken in case of severe adverse reactions.
You must carefully follow your doctor's instructions regarding the dose, infusion rate, and scheduling when infusing Cuvitru, so that the treatment works.
Form and route of administration
Selection of infusion sites:
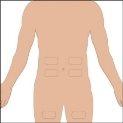
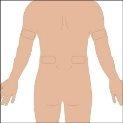
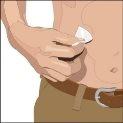
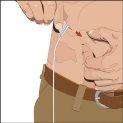
The suggested areas for subcutaneous infusion of Cuvitru are the abdomen, thighs, upper arms, or lower back. Cuvitru can be infused at several infusion sites. The infusion sites should be at least 10 cm apart. Avoid: bony areas, visible blood vessels, scars, and any area with inflammation (irritation) or infection. Alternate infusion sites with each administration, as instructed by your doctor or nurse.
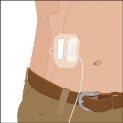
When using device-assisted infusion:
Several subcutaneous infusion sites can be used at the same time, with a set of multiple needles. The amount of product administered at a single site varies, and doses greater than 30 ml can be divided, as preferred.
When using manual administration:
Cuvitru can be administered using a syringe at a single infusion site. If administration at additional sites is required, a new sterile injection needle must be used.
The amount of product administered at a single site varies, and doses greater than 30 ml can be divided, as preferred.
Infusion rate:
Your doctor will determine the appropriate infusion technique and infusion rate for you, taking into account your individual dose, administration frequency, and product tolerability.
When using device-assisted infusion:
The recommended initial infusion rate is 10 ml per hour and per infusion site. If well tolerated, it can be increased at intervals of at least 10 minutes, up to a maximum of 20 ml per hour and per site, in the first two infusions. In subsequent infusions, the infusion rate can be increased according to tolerance.
If you have any further questions about using this medication, ask your doctor, pharmacist, or nurse.
When using manual administration:
Consult your doctor. Start with an infusion rate that does not cause discomfort. At no time should the infusion be painful. The maximum recommended infusion rate is approximately 1-2 ml per minute. Some infusion sites may tolerate larger infusion volumes than others.
If you have any further questions about using this medication, consult your doctor, pharmacist, or nurse.
In the following section, detailed instructions for use are provided:
Do not use Cuvitru at home until you have received instructions and training from your doctor or nurse.
Prepare the Cuvitru vials:
- Remove Cuvitru from the box. If the product is stored in a refrigerator, wait for the vials to reach room temperature. This may take up to 90 minutes.
- Do not apply heat or warm it in the microwave oven.
- Do not shake the vials.
| |
These include: Cuvitru vials, infusion materials (subcutaneous needle sets, transfer devices, syringes, sterile needle caps, sterile dressing, tape, gauze, sharps container, infusion pump (if used), tubes, infusion logbook).
|
|
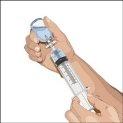
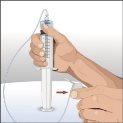
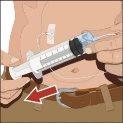
You must start the infusion immediately after introducing Cuvitru into the syringe. If administration is expected to take more than two hours, the dose should be divided and administered at different infusion sites. If Cuvitru remains in siliconized syringes for more than two hours, visible particles may form. If you use a sterile needle: connect a sterile syringe to the sterile needle and pull the syringe plunger to fill with air, which should be equivalent to the amount of solution you are extracting from the vial. Insert the needle into the center of the cap and inject air into the interior. Pull the plunger to extract the desired volume. |
|
|
|
|
|
|
|
| |
|
|
|
Use in children and adolescents
The same indications, doses, and infusion frequency for adults are valid for children and adolescents (from 0 to 18 years).
If you use more Cuvitru than you should
If you think you have used more Cuvitru than you should, consult your doctor as soon as possible.
If you miss a dose of Cuvitru
Do not administer a double dose of Cuvitru to make up for missed doses. If you think you have missed a dose, consult your doctor as soon as possible.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. These side effects may include chills, headache, dizziness, fever, vomiting, allergic reactions, nausea, joint pain, low blood pressure, and moderate lower back pain.
Some side effects, such as headache, chills, or body aches, can be mitigated by reducing the infusion rate.
Severe side effects
The infusion of medicines like Cuvitru can occasionally cause severe allergic reactions, although rare. You may experience a sudden drop in blood pressure and, in isolated cases, anaphylactic shock. Doctors are aware of these possible side effects and will monitor you during and after the initial infusions.
Immediately inform your doctor or nurse if you notice any of the following effects:
- dizziness, dizziness, or loss of consciousness;
- skin rash and itching, swelling of the mouth or throat, difficulty breathing, wheezing;
- abnormal heart rate, chest pain, bluish discoloration of the lips or fingers and toes;
- blurred vision.
When using Cuvitru at home, you can perform the infusion in the presence of your caregiver, who will help you monitor allergic reactions, stop the infusion, and seek help if necessary.
Also, see section 2 of this leaflet on the risk of allergic reactions and the use of Cuvitru at home.
The following side effects are very common (may affect more than 1 in 10 patients):
- headache;
- diarrhea and nausea;
- redness and pain at the infusion site;
- fatigue.
The following side effects are common (may affect up to 1 in 10 patients):
- dizziness, migraine, and somnolence;
- decreased blood pressure;
- abdominal pain;
- itching and skin rash;
- muscle pain;
- swelling, itching, skin rash, and bruising at the infusion site;
- pain.
The following side effects are uncommon (may affect up to 1 in 100 patients):
- burning sensation;
- pain in the lower abdomen;
- edema at the infusion site;
- positive antibody determination.
The frequency of the following side effects is unknown (cannot be estimated from the available data):
- Aseptic meningitis (inflammation of the layers covering the brain).
Side effects observed with similar medicines
The following side effects have been observed with the infusion of normal human immunoglobulin administered subcutaneously. Although these side effects have not been observed with Cuvitru, they may occur in people using it.
- tingling;
- tremor;
- increased heart rate;
- difficulty breathing;
- vocal cord dysfunction;
- chest pain;
- hardening or warmth at the infusion site.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are possible side effects not listed in this leaflet. You can also report them directly through the website of the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cuvitru
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the label and carton after EXP. The expiration date is the last day of the month indicated.
Do not use this medicine if you notice that the solution is cloudy, contains particles, or has changed color.
Store the vials in the outer packaging to protect them from light.
Do not store above 25°C.
Do not freeze.
If the product is stored in a refrigerator, the unopened vials should be removed from the refrigerator and placed at room temperature for at least 90 minutes before use. Do not use heating devices, including microwave ovens.
6. Container Content and Additional Information
Composition ofCuvitru
- The active ingredient is normal human immunoglobulin.
- 1 ml of Cuvitru contains 200 mg of human protein, of which at least 98% is immunoglobulin G (IgG).
The other components (excipients) are glycine and water for injectable preparations.
Appearance ofCuvitruand Container Content
Cuvitru is an injectable solution in vials of 5, 10, 20, or 40 ml. The solution is transparent and colorless, pale yellow or brown.
Each 5 ml vial contains: 1 g of normal human immunoglobulin
Each 10 ml vial contains: 2 g of normal human immunoglobulin
Each 20 ml vial contains: 4 g of normal human immunoglobulin
Each 40 ml vial contains: 8 g of normal human immunoglobulin
Each 50 ml vial contains: 10 g of normal human immunoglobulin
Package sizes:
1, 10, or 20 vials with 5 ml of injectable solution
1, 10, 20, or 30 vials with 10 ml of injectable solution
1, 10, 20, or 30 vials with 20 ml of injectable solution
1, 5, 10, or 20 vials with 40 ml of injectable solution
1 vial with 50 ml of injectable solution
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer:
Marketing Authorization Holder:
Baxalta Innovations GmbH
Industriestrasse 67
1221 Vienna
Austria
Manufacturer:
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
7860 Lessines
Belgium
Local Representative:
Takeda Pharmaceutical Spain S.A.
Calle Albacete, 5, 9th floor
Edificio Los Cubos
28027 Madrid
Spain
Tel: +34 91 790 42 22
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria: Cuvitru 200 mg/ml injection solution for subcutaneous use
Belgium, France: Cuvitru 200 mg/ml solution for subcutaneous injection
Czech Republic, Denmark, Finland, Germany, Greece, Italy, Norway, Poland: Cuvitru
Ireland, United Kingdom: Cuvitru 200 mg/ml solution for subcutaneous injection
Netherlands: Cuvitru 200 mg/ml, solution for subcutaneous injection
Slovakia, Sweden: Cuvitru 200 mg/ml
Spain: Cuvitru 200 mg/ml, subcutaneous injectable solution
Date of the last revision of this leaflet:January 2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CUVITRU 200 MG/ML SOLUTION FOR SUBCUTANEOUS INJECTIONDosage form: INJECTABLE, 165 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Octapharma S.A.Prescription requiredDosage form: INJECTABLE, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 200 mg human plasma protein/ mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Csl Behring GmbhPrescription required
Online doctors for CUVITRU 200 MG/ML SOLUTION FOR SUBCUTANEOUS INJECTION
Discuss questions about CUVITRU 200 MG/ML SOLUTION FOR SUBCUTANEOUS INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions