
Cutaquig

Ask a doctor about a prescription for Cutaquig

How to use Cutaquig
Leaflet accompanying the packaging: Information for the user
CUTAQUIG 165 mg/mlsolution for injection
Human normal immunoglobulin (SCIg)
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Cutaquig and what is it used for
- 2. Important information before using Cutaquig
- 3. How to use Cutaquig
- 4. Possible side effects
- 5. How to store Cutaquig
- 6. Contents of the packaging and other information
1. What is Cutaquig and what is it used for
What is Cutaquig
Cutaquig belongs to a class of medicines known as "normal human immunoglobulins".
Immunoglobulins are also called antibodies; they are proteins found in the blood of healthy people.
Antibodies are part of the immune system (the body's natural defense mechanism) and help the body fight infections.
How Cutaquig works
Cutaquig contains immunoglobulins that have been obtained from the blood of healthy people. This medicine works in the same way as the immunoglobulins that are naturally present in the blood.
What is Cutaquig used for
Cutaquig is used in patients who do not have enough antibodies to fight infections and therefore often get infections. Regular administration of the appropriate dose of Cutaquig can increase the abnormally low concentration of immunoglobulins in the patient's blood to a normal level (replacement therapy).
Cutaquig is prescribed for adults and children (aged 0-18) in the following situations:
Treatment of patients born with reduced or absent ability to produce immunoglobulins (primary immunodeficiency).
Patients with acquired antibody deficiency (secondary immunodeficiency) due to certain diseases or treatments, who experience severe or recurrent infections.
2. Important information before using Cutaquig
When not to use Cutaquig:
- if the patient is allergic to normal human immunoglobulin or any of the other ingredients of this medicine (listed in section 6).
DO NOT inject Cutaquig into blood vessels.
Warnings and precautions:
Before starting treatment with Cutaquig, discuss it with your doctor or pharmacist.
The patient may be allergic (hypersensitive) to immunoglobulins even without knowing it.
Actual allergic reactions, such as a sudden drop in blood pressure or anaphylactic shock (a sudden drop in blood pressure combined with other symptoms such as throat swelling, difficulty breathing, and skin rash) are rare, but may occur, even if the patient has previously tolerated human immunoglobulins well. This may happen especially if the patient's blood does not have enough immunoglobulin A (IgA deficiency) and has antibodies against IgA.
- Before treatment, tell your doctor or other healthcare professional if you have an IgA deficiency. Cutaquig contains a minimal amount of IgA, which may cause an allergic reaction. In such rare cases, allergic reactions such as a sudden drop in blood pressure or shock (see also section 4) may occur. The symptoms and signs of such rare allergic reactions are as follows:
- dizziness, dizziness, or fainting,
- skin rash and itching, swelling of the mouth or throat, difficulty breathing, wheezing,
- abnormal heart rate, chest pain, blue discoloration of the lips or fingers and toes,
- blurred vision. If the patient notices such symptoms during the infusion of Cutaquig, they should immediately tell their doctor. The doctor will decide whether to reduce the infusion rate or stop it altogether.
- Tell your doctor if you have had heart or blood vessel disease, or blood clots, or if you have been immobilized for some time. It is known that such circumstances increase the risk of blood clots after using Cutaquig. You should also tell your doctor about all medicines you are currently taking, as certain medicines, such as those containing the estrogen hormone (e.g., birth control pills), may increase the risk of blood clots. If you experience symptoms or signs such as shortness of breath, chest pain, pain and swelling of a limb, or weakness or numbness of one side of the body after using Cutaquig, you should contact your doctor immediately.
- If you experience symptoms or signs such as severe headache, neck stiffness, drowsiness, fever, photophobia, nausea, and vomiting after using Cutaquig, you should contact your doctor. These may be symptoms of aseptic meningitis. The doctor will decide whether additional tests are necessary and whether to continue using Cutaquig.
- Cutaquig contains antibodies against blood groups, which can cause destruction of red blood cells and thus anemia (too few red blood cells).
The doctor will reduce the risk of possible complications by ensuring:
- that the patient is not allergic to normal human immunoglobulin. At the beginning, the medicine must be administered at a slow infusion rate. The recommended infusion rates given in section 3 must be strictly followed.
- that the patient is carefully monitored for any symptoms throughout the infusion, especially if: the patient is receiving normal human immunoglobulin for the first time, the patient has recently changed from another medicine to Cutaquig, a long time has passed since the last infusion (more than eight weeks).
In such cases, it is recommended to monitor the patient during the first infusion and for one hour after its completion. If the above points do not apply to the patient, it is recommended to observe them for at least 20 minutes after administration.
Children and adolescents
The above warnings and precautions apply to both adults and children.
Cutaquig and other medicines
- Tell your doctor or pharmacist about all medicines you are currently taking, or have recently taken, or plan to take.
- Do not mix Cutaquig with any other medicine.
- Before vaccination, tell the vaccinating doctor about treatment with Cutaquig. Cutaquig (like all other medicines containing normal human immunoglobulin) may interfere with the effectiveness of certain live virus vaccines, such as measles, rubella, mumps, and chickenpox vaccines. Therefore, after using Cutaquig, the patient may need to wait up to 3 months before receiving a live attenuated vaccine. In the case of measles vaccination, such impairment of effectiveness may last for up to one year.
- Blood glucose testing Some types of systems used to measure blood glucose (so-called glucometers) incorrectly recognize maltose contained in Cutaquig as glucose. This can lead to falsely elevated blood glucose readings during infusion and for about 15 hours after the end of the infusion, and consequently to inappropriate use of insulin resulting in life-threatening hypoglycemia (i.e., low blood sugar level). Also, in cases of true hypoglycemia, treatment may not be given due to falsely changed elevated glucose readings. Therefore, when administering Cutaquig or other products containing maltose, blood glucose should be measured using a system that uses a glucose-specific method. Systems based on glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose oxidoreductase should not be used. You should carefully read the information about the blood glucose measurement system, including test strips, to determine if the system is suitable for testing during concurrent use of parenterally administered maltose-containing drugs. In case of doubts, you should contact the treating doctor to determine if the blood glucose measurement system is suitable for testing during concurrent use of parenterally administered maltose-containing drugs.
Cutaquig with food, drink, and alcohol
No interactions have been observed.
Pregnancy, breastfeeding, and fertility
In pregnancy and breastfeeding, or if you are pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine. This medicine should be used during pregnancy and breastfeeding only after consulting a doctor or pharmacist.
Clinical studies of Cutaquig in pregnant women have not been conducted. However, medicines containing immunoglobulins have been used in pregnant and breastfeeding women for many years, and no adverse effects on the course of pregnancy or on the fetus or newborn have been observed.
If a breastfeeding patient is taking Cutaquig, the immunoglobulins contained in this medicine will also pass into breast milk. This will protect the child from certain infections.
Experience with immunoglobulins does not indicate a harmful effect on fertility.
Driving and using machines
Certain side effects associated with Cutaquig may impair the ability to drive and use machines. Patients who experience side effects during treatment should wait for them to resolve before driving or using machines.
Cutaquig contains sodium
This medicine contains 33.1 mg of sodium (the main component of common salt) per 48 ml vial and 13.8 mg of sodium per 20 ml vial. This is equivalent to 1.7% and 0.7%, respectively, of the recommended maximum daily sodium intake in the diet for adults.
Information about the origin of Cutaquig
Cutaquig is obtained from human blood plasma (the liquid component of blood). When medicines are made from human blood or plasma, certain precautions are taken to prevent the transmission of infections to patients. These include:
- careful selection of blood and plasma donors to exclude donors who may be carriers of infections,
- testing each donation and pool of collected plasma for the presence of viruses/infections,
- including steps in the blood or plasma processing process aimed at inactivating or removing viruses. Despite these precautions, when administering medicines prepared from human blood or plasma, it cannot be completely excluded that the possibility of transmitting an infection, including unknown or newly emerging viruses or other types of infections.
The measures taken are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV - the virus that causes AIDS), hepatitis B virus, and hepatitis C virus.
The measures taken may have limited effectiveness against non-enveloped viruses, such as hepatitis A virus and parvovirus B19.
No association has been found between immunoglobulin products and viral hepatitis A or parvovirus B19 infection, possibly because the antibodies against these infections contained in this product have a protective effect.
It is strongly recommended that in each case of administering Cutaquig to a patient, the name and batch number of the medicine be recorded to document the batch used (see also Annex I: Administration guidelines).
3. How to use Cutaquig
This medicine should always be used exactly as prescribed by your doctor or pharmacist. In case of doubts, consult your doctor or pharmacist.
Cutaquig must be administered by subcutaneous infusion.
Treatment will be initiated by the patient's doctor or a nurse experienced in treating patients with weakened immune systems.
Once the doctor/nurse has determined the appropriate dose and infusion rate for the patient and the patient has received several initial infusions under supervision, the patient may be allowed to self-administer the treatment at home or have it administered at home by a trained caregiver. The doctor or nurse experienced in managing patients using home treatment will ensure that the patient or their caregiver receives training and detailed information on:
- the principle of performing infusions under aseptic conditions,
- the use of an infusion device (if necessary),
- keeping a treatment diary,
- actions to be taken in case of serious side effects (see also section 4). As soon as the patient is able to self-administer the treatment, and no side effects occur during treatment, the doctor will allow the patient to continue treatment at home.
Dosage
The dosage and infusion rate for an individual patient will be determined by the doctor, who will adjust the dose based on the patient's body weight, previous treatment received by the patient, and their response to treatment. Always follow the doctor's instructions.
Replacement therapy in primary immunodeficiency syndromes:
The doctor will determine whether the patient requires a loading dose (for adults and children) of at least 1.2 to 3.0 ml/kg body weight divided over several days. Then, the patient will receive Cutaquig at regular intervals, from daily to every two weeks.
The cumulative dose administered over a month will be approximately 2.4 to 4.8 ml/kg body weight. The doctor or nurse may adjust the dose based on the patient's response to treatment.
Replacement therapy in secondary immunodeficiency:
The recommended dose of Cutaquig is a cumulative monthly dose of 1.2-2.4 ml/kg administered at equal intervals (about once a week). It may be necessary to inject each dose into a different site. The doctor may adjust the dose based on the patient's response to treatment.
Do not change the dose or frequency of administration of Cutaquig without consulting your doctor. If you think you should receive Cutaquig more or less frequently, discuss it with your doctor. If you think you have missed a dose, contact your doctor as soon as possible.
Method and route of administration
Choice of infusion site (s):
Suggested areas for subcutaneous infusion of Cutaquig are the abdomen, thighs, upper arms, and upper legs/buttocks. Infusion can be administered at more than one site simultaneously.
There is no limit to the number of infusion sites, but individual sites should be at least 5 cm apart. During each administration, change the infusion sites as recommended by the doctor or nurse.
Volume of infusion administered at one site is variable, but it is recommended to divide large infusion volumes (> 30 ml) and administer them at multiple different sites. In newborns and children, infusion sites can be changed after administering 5-15 ml.
Infusion rate:
The doctor will determine the appropriate infusion rate for the patient, taking into account the individual dose, dosing frequency, and product tolerance.
The recommended initial infusion rate is 15 ml/hour/site if the patient has not been previously treated with SCIG. For a patient already treated with SCIG and switching to Cutaquig, it is recommended to use the previously used infusion rate in the initial infusions. In subsequent infusions, if the medicine is well tolerated, the infusion rate can be gradually increased by approximately 10 ml/hour/site every 2-4 weeks in adults (≥40 kg) and up to 10 ml/hour/site every 4 weeks in children and adolescents (<40 kg).
Then, if the patient tolerates the initial infusions at full dose per site and maximum rate, it is possible to consider increasing the rate of subsequent infusions up to a maximum flow rate of 67.5 ml/hour/site in adults (≥40 kg) and 25 ml/hour/site in children and adolescents (<40 kg).
Detailed instructions for use are given below.
Cutaquig is intended for subcutaneous use only (under the skin). Do not inject the medicine into blood vessels.
Cutaquig can be used at home only after receiving proper instructions and training from a doctor or nurse.
When using Cutaquig, follow each point of the administration guidelines attached at the end of this leaflet (Annex I) and use aseptic techniques (sterile technique).
Wear gloves if the doctor recommends wearing them when preparing the infusion.
Use in children and adolescents
In children and adolescents (from 0 to 18 years), the same indications, doses, and infusion frequencies apply as in adults.
Using a higher dose of Cutaquig than recommended
If you think you have administered too much Cutaquig, contact your doctor or nurse as soon as possible.
Missing a dose of Cutaquig
If you miss a dose, tell your doctor or nurse as soon as possible. Do not administer a double dose of Cutaquig to make up for a missed dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, such as chills, headache, dizziness, fever, vomiting, allergic reactions, nausea, joint pain, low blood pressure, and moderate back pain, although not everybody gets them.
Certain side effects, such as headache, chills, or body pain, can be limited by reducing the infusion rate.
In clinical trials evaluating the safety of Cutaquig, no serious side effects of the medicine were observed in patients who received it.
The patient may be allergic (hypersensitive) to immunoglobulins and may experience allergic reactions, such as a sudden drop in blood pressure or, in single cases, shock. Doctors are aware of these potential side effects and will monitor the patient during the first few infusions and after their completion.
If the patient experiences any of the following symptoms, they should immediately tell their doctor:
- dizziness, dizziness, or fainting,
- skin rash and itching, swelling of the mouth or throat, difficulty breathing, wheezing,
- abnormal heart rate, chest pain, blue discoloration of the lips or fingers and toes,
- blurred vision. If Cutaquig is used at home, the patient may perform the infusion in the presence of a caregiver who will observe the patient for signs of an allergic reaction. If any symptoms of an allergic reaction occur, the infusion should be stopped and help should be sought if necessary. Information about the risk of allergic reactions is also given in section 2 of this leaflet. The following side effects occur very frequently (may occur in more than 1 in 10 infusions):
- Reactions at the infusion site, such as redness, swelling, itching, and discomfort.
The following side effects occur uncommonly (may occur in more than 1 in 1000 infusions to less than 1 in 100 infusions):
- Headache
- Nausea
- Fatigue
The following side effects occur rarely (may occur in more than 1 in 10,000 infusions):
- Dizziness
- Abdominal pain
- Abdominal distension
- Vomiting
- Nausea
- Muscle pain
- Joint pain
- Fever
- Chills
- Chest discomfort
- Flu-like symptoms
- Pain
- General malaise
- Positive blood test result for antibodies
- Abnormal blood test results indicating destruction of red blood cells.
- Increased hemoglobin levels
- Increased creatinine levels in the blood
- Rash
- Skin reactions
- Increased activity of certain liver enzymes called transaminases
Other side effects that did not occur in clinical trials but have also been reported include:
- Hypersensitivity (e.g., flushing, urticaria)
- Increased blood pressure
- Problems related to blood clot formation in blood vessels (e.g., deep vein thrombosis, stroke)
- Blood clots in blood vessels (see also section 2 "Warnings and precautions")
- Itching
- Back pain
Side effects observed with similar medicines
The following additional side effects have been observed with subcutaneous infusion of normal human immunoglobulin. It is possible that they may occur in some patients using this medicine.
- chills,
- pallor,
- diarrhea,
- pain at the injection site,
- rapidly progressing redness of the neck/face,
- feeling of heat,
- feeling of cold,
- weakness,
- throat constriction,
- difficulty breathing,
- asthma-like symptoms,
- cough,
- facial swelling,
- a syndrome called "aseptic meningitis" (see also section 2 "Warnings and precautions").
If the patient experiences any of the following symptoms, they should immediately tell their doctor: These may be symptoms of a serious problem.
- Severe headache accompanied by symptoms such as nausea, vomiting, neck stiffness, fever, and photophobia. These may be symptoms of transient and reversible non-infectious inflammation of the membranes surrounding the brain and spinal cord (meningitis).
- Pain, swelling, increased temperature, redness, or a lump in the arm or leg, shortness of breath without apparent cause, pain or discomfort in the chest that worsens with deep breathing, rapid heart rate without apparent cause, numbness or weakness of one side of the body, sudden confusion or difficulty speaking. These may be symptoms of a blood clot.
Such side effects may occur even if the patient has previously received human immunoglobulins and tolerated them well.
Additional information about circumstances that increase the risk of side effects is given in section 2.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the national reporting system:
Department for Monitoring of Adverse Reactions to Medicinal Products
Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Email: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Cutaquig
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after "EXP". The expiry date refers to the last day of that month.
Store in a refrigerator (2°C - 8°C). Do not freeze. Store the vial in the outer carton to protect from light.
Within the shelf-life, the medicine can be stored at room temperature (not above 25°C) for up to 9 months without the need for refrigeration during this period; if the medicine is not used within this period, it must be discarded after its expiration.
After first opening, the medicine should be used immediately.
Do not use Cutaquig if the solution is cloudy or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Cutaquig contains
The active substance is human normal immunoglobulin at a concentration of 165 mg/ml (at least 95% is immunoglobulin G).
- IgG ……….. 71%
- IgG ……….. 25%
- IgG ……….. 3%
- IgG ………… 2%
Other ingredients are: maltose, polysorbate 80, and water for injections.
The maximum IgA content is 300 micrograms/ml.
Cutaquig contains sodium in an amount of ≤ 30 mmol/l.
What Cutaquig looks like and contents of the pack
Cutaquig is a solution for injection.
The solution is clear and colorless.
During storage, the solution may become slightly opalescent and pale yellow.
Cutaquig is available in:6, 10, 12, 20, 24, or 48 ml solution in a vial (type I glass) with a bromobutyl rubber stopper - pack sizes containing 1, 10, or 20 vials.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Octapharma (IP) SPRL
65 Allée de la Recherche
1070 Anderlecht
Belgium
Manufacturer:
Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Strasse 235
1100 Vienna
Austria
Octapharma AB
Lars Forssells gata 23
112 75 Stockholm
Sweden
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Estonia,
Finland, France, Spain, Netherlands, Ireland, Iceland, Lithuania,
Latvia, Luxembourg, Malta, Germany, Norway, Poland,
Portugal, Romania, Slovakia, Slovenia, Sweden, Hungary,
United Kingdom (Northern Ireland), Italy:
Cutaquig
Date of last revision of the leaflet: 11/2023
Annex I – Administration guidelines
1. Prepare the required number of Cutaquig vials
- If the vials are stored in the refrigerator, remove them to room temperature at least 90 minutes before the infusion.
- Do not heat the vials or place them in a microwave oven.
- Do not shake the vials to avoid foaming.
2. Preparation of the infusion
- Choose and prepare a clean work area using antiseptic wipes or a disinfectant solution (Figure 1).
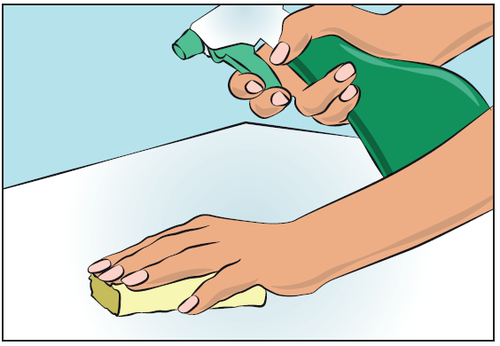
Figure 1
- Prepare the infusion equipment and materials:
- infusion pump (optional) and compatible syringe (or syringes),
- needle (for withdrawing the product from the vial),
- infusion set,
- infusion tubing and Y-connector (if needed),
- alcohol and alcohol swabs/antiseptic wipes,
- gauze or transparent dressing and adhesive tape (without dressing),
- sharps container,
- treatment diary and pen.
- Wash your hands thoroughly and wait for them to dry (Figure 2). Use a disinfectant gel as shown in the training.
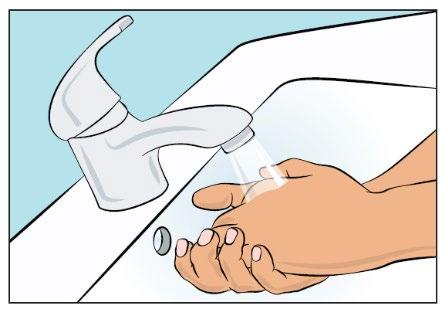
Figure 2
- If necessary, program the pump according to the manufacturer's instructions and as shown in the training provided by the doctor or nurse.
3. Checking and opening the vials
- Check each vial carefully:
- for the correct dose, as prescribed by the doctor,
- the appearance of the solution (should be clear and colorless to pale yellow or light brown),
- that the protective cap is in place and intact,
- the expiry date and batch number.
- Do not use the solution if it is cloudy or contains particles.
- Remove the protective cap.
- Disinfect the rubber stopper with an antiseptic wipe and wait for it to dry (Figure 3).
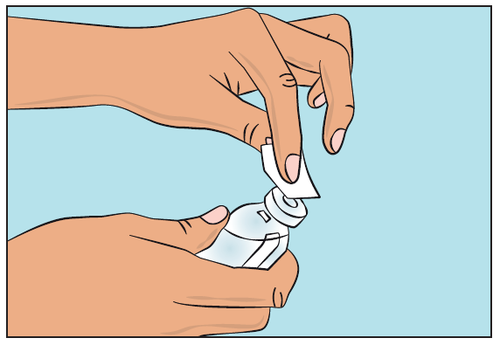
Figure 3
4. Preparation and filling of the syringe
- Open the packaging containing a sterile syringe and needle.
- Screw the needle onto the syringe.
- Draw the plunger back to fill the syringe with a volume of air approximately equal to the volume of the solution to be withdrawn from the vial.
- Insert the needle into the vial and turn the vial upside down. Inject the air, making sure the needle tip is above the solution, to avoid foaming.
- Then slowly withdraw the required volume of Cutaquig, ensuring the needle tip remains in the solution (Figure 4).
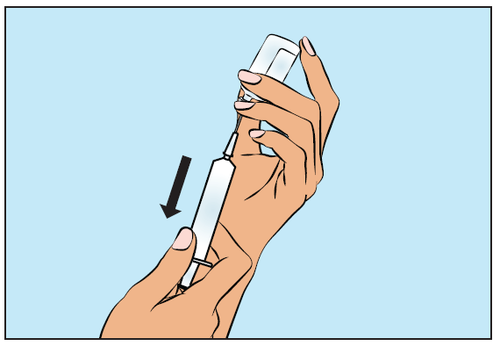
Figure 4
- Withdraw the needle from the vial.
- It may be necessary to repeat this procedure if the calculated dose requires withdrawal of the product from more than one vial.
- After completion, detach the needle and discard it in the sharps container.
- Proceed immediately to the next step, as the IgG solution should be used as soon as possible.
5. Preparation of the infusion pump and tubing (optional)
- Prepare the pump according to the manufacturer's instructions.
- To pre-fill the infusion tubing, connect the filled syringe to the infusion tubing and gently press the plunger to fill the tubing with Cutaquig and remove all air from it (Figure 5).
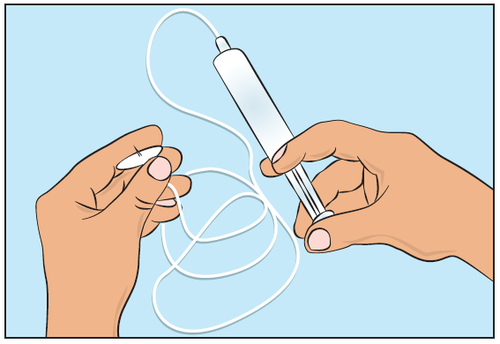
Figure 5
6. Selection of infusion sites and insertion of the needle (s) for infusion
- Cutaquig infusions can be administered in the following areas: abdomen, thighs, upper arms, and upper legs/buttocks (Figure 6).
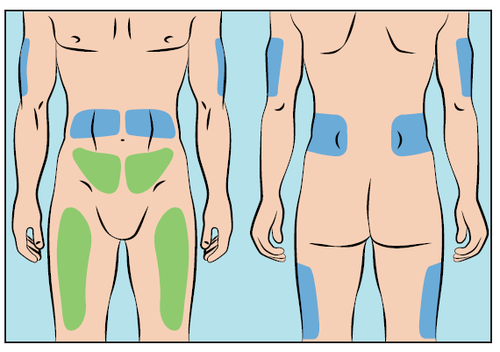
Figure 6
- Individual infusion sites should be at least 5 cm apart.
- Use infusion sites other than those used for the previous administration.
- Avoid inserting the needle into scars, tattoos, stretch marks, and damaged/infected/red skin areas.
- Clean the skin at the chosen infusion site (s) with an antiseptic wipe and wait for it to dry.
- Pinch the skin fold around the infusion site between the thumb and index finger (Figure 7), carefully remove the needle cover, and insert the needle into the skin (Figure 8). The angle of needle insertion depends on the type of infusion set used.
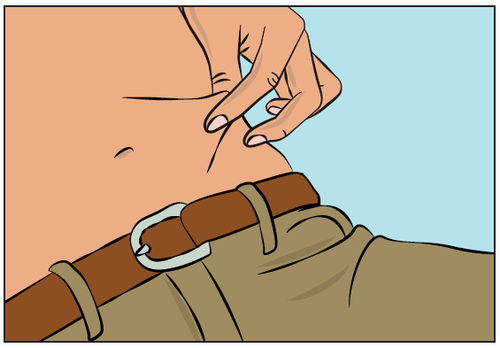
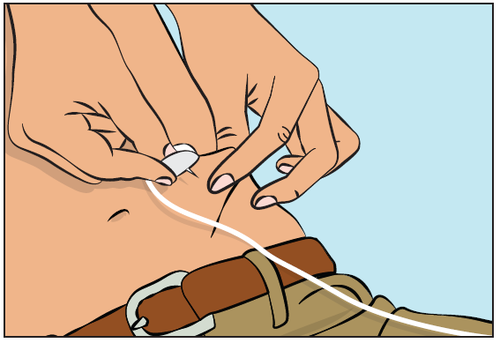
Figure 7
Figure 8
7. Checking the infusion
- Do not administer the solution into a blood vessel.
- Secure the needle in place using a sterile gauze and adhesive tape or a transparent dressing (Figure 9).
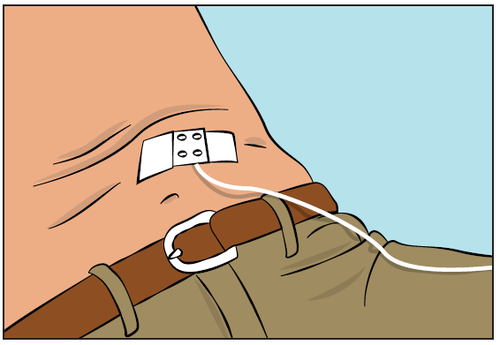
Figure 9
8. Starting the infusion
- Start the infusion. If the medicine is administered through an infusion pump, follow the manufacturer's instructions.
9. Recording the infusion
- On each vial of Cutaquig, there is a detachable part of the label with batch number information. Attach this label to the patient's treatment diary or infusion record. Record information about the dose, date, time, infusion site location, and any infections or side effects, as well as any notes related to the infusion.
10. After completing the infusion
- Gently pull the needle (s) out of the skin and immediately discard it in the sharps container.
- If necessary, apply a small gauze to the needle site and apply a dressing.
- Discard all used disposable materials, as well as any unused medicine and empty vials, as instructed by the doctor or nurse and in accordance with local requirements. Clean and store all reusable equipment (e.g., pump) in a safe place until the next infusion.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterOctapharma AB Octapharma GmbH Octapharma Pharmazeutika Produktionsges.m.g.H.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CutaquigDosage form: Solution, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Baxalta Belgium Manufacturing S.A.Prescription requiredDosage form: Solution, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Instituto Grifols, S.A.Prescription requiredActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Baxalta Belgium Manufacturing S.A.Prescription required
Alternatives to Cutaquig in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cutaquig in Spain
Alternative to Cutaquig in Ukraine
Online doctors for Cutaquig
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cutaquig – subject to medical assessment and local rules.














