
COPAXONE 40 mg/mL injectable solution in pre-filled syringe

How to use COPAXONE 40 mg/mL injectable solution in pre-filled syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Copaxone 40 mg/ml solution for injection in pre-filled syringe
glatiramer, acetate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Copaxone and what is it used for
- What you need to know before you use Copaxone
- How to use Copaxone
- Possible side effects
- Storing Copaxone
- Contents of the pack and further information
1. What is Copaxone and what is it used for
Copaxone is a medicine, indicated for the treatment of relapsing forms of multiple sclerosis (MS). It modifies the way your body's immune system works and is classified as an immunomodulatory agent. The symptoms of multiple sclerosis (MS) are caused by a defect in your body's immune system. This produces areas of inflammation in the brain and spinal cord.
Copaxone is used to reduce the number of times you have MS attacks (relapses). It has not been shown to help if you have a form of MS that does not have relapses, or has very few relapses. Copaxone may not have any effect on the duration of an MS attack, or how bad you feel during an attack.
2. What you need to know before you use Copaxone
Do not use Copaxone
- if you are allergic to glatiramer acetate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Copaxone may cause severe allergic reactions, some of which can be life-threatening.
These reactions can occur shortly after administration, even months or years after the start of treatment, and even if no allergic reactions have occurred after previous administrations.
The signs and symptoms of allergic reactions can overlap with post-injection reactions. Your doctor will inform you about the signs of an allergic reaction.
Consult your doctor or pharmacist before starting Copaxone if you have any kidney or heart problems, as you may need to have regular tests or check-ups.
Consult your doctor or pharmacist before starting Copaxone if you have or have had liver problems (including those caused by alcohol consumption).
Children
Copaxone must not be used in children under 18 years of age.
Elderly patients
Copaxone has not been studied specifically in elderly patients. Please consult your doctor.
Using Copaxone with other medicines
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor about the treatment with Copaxone during pregnancy.
Copaxone may be used during pregnancy under the guidance of your doctor.
Limited human data do not show negative effects of Copaxone on newborns/infants. Copaxone may be used during breastfeeding.
Driving and using machines
It is not known if Copaxone has an influence on the ability to drive or use machines.
3. How to use Copaxone
Follow the instructions for administration of this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist again.
The recommended dose in adults is one pre-filled syringe (40 mg of glatiramer acetate), administered under the skin (subcutaneously) three times a week, with at least 48 hours between injections, for example, on Monday, Wednesday, and Friday. It is recommended to administer the medicine on the same days every week.
It is very important that the injection of Copaxone is done correctly:
- Only under the skin (subcutaneous use) (see "Instructions for use")
- At the dose indicated by your doctor. Administer only the dose prescribed by your doctor.
- Never use the same syringe more than once. Any unused or leftover product should be discarded.
- Do not mix or co-administer the contents of Copaxone pre-filled syringes with any other product.
- If the solution contains particles, do not use it. Use a new syringe.
The first time you use Copaxone, you will be given complete instructions and will be supervised by a doctor or nurse. They will be with you during the injection and half an hour later, just to make sure you have no problems.
Instructions for use
Read these instructions carefully before using Copaxone.
Before injection, make sure you have everything you need:
- A blister pack containing a pre-filled syringe of Copaxone solution for injection.
- A container for disposing of used needles and syringes.
- For each injection, take only one blister pack containing a pre-filled syringe from the packaging. Keep the rest of the syringes in the box.
- If your syringe was in the refrigerator, take the blister pack containing the syringe out at least 20 minutes before you are going to inject the medicine, so it can warm up to room temperature.
Wash your hands thoroughly with soap and water.
If you want to use an injection device to inject yourself, the CSYNC device can be used with Copaxone. The CSYNC device is only authorized for use with COPAXONE and has not been tested with other products. Please consult the instructions for use provided with the CSYNC injection device.
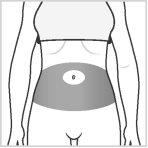
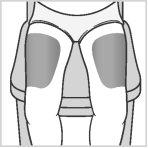
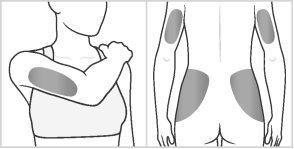
Choose an injection site within the areas, following the diagrams.
There are seven possible injection areas on your body:
Area 1: Abdomen area around the navel. Avoid putting the injection 5 cm to either side of the navel
Areas 2 and 3: Thighs (above the knees)
Areas 4, 5, 6, and 7: Upper arms, and upper hips (below the waist)
Within each injection area, there are multiple injection sites. Choose a different site for each injection. This will reduce the chance of irritation or pain at the injection site. Change the injection areas and also change the injection site within each area. Do not always use the same injection site.
Warning: Do not inject into any area that is painful or discolored, or where you notice lumps or hard nodules.
It is recommended to have a plan with the injection sites scheduled and to write it down in a diary. There are some areas on your body that may be difficult for self-injection (such as the back of your arm). If you want to use them, you may need help.
How to inject:
- Take the syringe out of the protective blister pack by peeling off the blister cover.
- Remove the needle cap, do notremove the protector with your mouth or teeth.
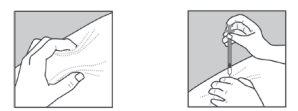
- Gently pinch the skin, making a fold between your thumb and index finger of your free hand (Figure 1).
- Gently insert the needle into the skin as shown in Figure 2.
- Inject the medicine by pushing the plunger firmly until it stops, leaving the syringe empty.
- Remove the syringe and needle.
- Dispose of the syringe in a secure container for sharps. Do not throw away used syringes in the household waste, put them carefully in a puncture-proof container as recommended by your doctor or nurse.
Figure 1 Figure 2
If you feel that the effect of Copaxone is too strong or too weak, tell your doctor.
If you use more Copaxone than you should
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 915620420, indicating the medicine and the amount ingested.
If you forget to use Copaxone
Administer it as soon as you remember or can administer it, and omit it the next day. Do not administer a double dose to make up for forgotten doses. If possible, you should return to your regular administration schedule the following week.
If you stop using Copaxone
Do not stop using Copaxone without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions (hypersensitivity, anaphylactic reaction)
You may develop a severe allergic reaction to this medicine shortly after administration. This is a rare side effect. These reactions can occur months or years after the start of treatment with Copaxone, even if no allergic reactions have occurred after previous administrations.
If you notice any of the following side effects, stop using Copaxone and contact your doctor immediately, or go to the emergency department of the nearest hospital:
- Widespread rash (red spots or hives)
- Swelling of the eyelids, face, lips, mouth, throat, or tongue
- Sudden shortness of breath, difficulty breathing, or wheezing
- Seizures (fits)
- Difficulty swallowing or speaking
- Fainting (syncope), feeling dizzy or faint
- Collapse
Other post-injection reactions (reaction immediately after injection)
Some people may have one or more of the following symptoms minutes after the injection of Copaxone. These usually do not pose a problem and disappear within half an hour.
However, if the following symptoms last more than 30 minutes, contact your doctor immediately, or go to the emergency department of the nearest hospital:
- Flushing (redness) of the chest or face (vasodilation)
- Difficulty breathing (dyspnea)
- Chest pain
- Fast or strong heartbeats (palpitations, tachycardia)
Liver problems
Liver problems or worsening of liver problems, including liver failure (which in some cases led to liver transplantation), can rarely occur with Copaxone. Contact your doctor immediately if you have symptoms such as:
- Nausea
- Loss of appetite
- Dark-colored urine and pale stools
- Yellowing of the skin or the white of the eye
- Bleeding more easily than normal
Generally, the following side effects reported by patients treated with Copaxone 40 mg/ml three times a week were also reported in patients treated with Copaxone 20 mg/ml (see the following list).
Very common: may affect more than 1 in 10 people
- Infections, flu
- Anxiety, depression
- Headache
- Nausea
- Skin rash
- Pain in the joints or back
- Feeling weak, skin reactions at the injection site including redness of the skin, pain, blistering, itching, swelling of the tissues, inflammation, and hypersensitivity (these reactions at the injection site are not abnormal and usually disappear over time), non-specific pain.
Common: may affect up to 1 in 10 people
- Respiratory tract inflammation, gastric disease, fever, ear inflammation, runny nose, dental abscesses, vaginal candidiasis
- Non-malignant skin growths (benign skin neoplasms), tissue growth (neoplasia)
- Swollen lymph nodes
- Allergic reactions
- Loss of appetite, weight gain
- Nervousness
- Altered sense of taste, increased muscle tone, migraine, speech problems, fainting, tremor
- Double vision, eye problems
- Hearing problems
- Cough, hay fever
- Anal or rectal problems, constipation, tooth decay, indigestion, difficulty swallowing, intestinal incontinence, vomiting
- Abnormal liver function test results
- Bruises, excessive sweating, itching, skin changes, hives
- Neck pain
- Need to urinate urgently, frequent urination, inability to empty the bladder properly
- Chills, facial swelling, loss of tissue under the skin at the injection site, local reaction, peripheral swelling due to fluid accumulation, fever.
Uncommon: may affect up to 1 in 100 people
- Abscesses, inflammation of the skin and soft tissue, boils, herpes, kidney inflammation
- Skin cancer
- Increased white blood cell count, decreased white blood cell count, enlarged spleen, decreased platelet count, change in the shape of white blood cells
- Enlarged thyroid, overactive thyroid
- Low alcohol tolerance, gout, increased blood fat levels, increased sodium in the blood, decreased ferritin in the blood
- Strange dreams, confusion, euphoric state, seeing, hearing, smelling, touching, or feeling things that are not there (hallucinations), aggression, abnormally happy state, personality disorders, suicidal attempt
- Numbness of the hands and pain (carpal tunnel syndrome), mental disorders, seizures (fits), problems with writing and reading, muscle disorders, movement problems, muscle spasms, nerve inflammation, abnormal nerve-muscle connection causing abnormal muscle function, rapid involuntary eye movements, paralysis, foot drop (peroneal nerve paralysis), state of unconsciousness (stupor), blind spots
- Cataracts, corneal lesions, dry eyes, eye bleeding, drooping eyelid, dilated pupil, optic nerve damage causing vision problems
- Fast heartbeats, slow heartbeats, episodes of fast heartbeats
- Varicose veins
- Periodic breathing stops, nosebleeds, abnormally rapid or deep breathing (hyperventilation), feeling of throat constriction, lung problems, inability to breathe due to throat constriction (feeling of suffocation)
- Inflammation of the small intestine, colon polyps, intestinal inflammation, belching, rectal problems, tooth decay, indigestion, difficulty swallowing, intestinal incontinence, vomiting
- Gallstones, enlarged liver
- Skin and soft tissue swelling, skin rash due to contact, painful red lumps on the skin, lumps on the skin
- Swelling, inflammation, and pain of the joints (arthritis or arthrosis), inflammation and pain of the fluid-filled sacs that cover the joints (existing in some of the joints), side pain, decreased muscle mass
- Blood in the urine, kidney stones, urinary system problems, urine abnormalities
- Breast swelling, difficulty getting an erection, prolapse of the pelvic organs, prolonged erections, prostate problems, abnormal Pap smear (abnormal cervical smear), testicular problems, vaginal bleeding, vaginal disorders
- Cysts, hangover, lower than normal body temperature (hypothermia), non-specific inflammation, tissue destruction at the injection site, problems with the mucous membranes
- Changes after vaccination
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Copaxone
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton (EXP). The expiry date is the last day of the month indicated.
Store in a refrigerator (between 2 °C and 8 °C).
Copaxone prefilled syringe solution for injection may be stored for up to one month at room temperature (between 15 °C and 25 °C). This can only be done once. If after this one-month period the Copaxone prefilled syringes have not been used and are still in their original packaging, they should be returned to the refrigerator.
Do not freeze.
The prefilled syringes should be stored in their original packaging, protected from light.
Discard any syringe that contains particles.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the SIGRE collection point at your pharmacy. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Composition ofCopaxone
- The active substance is glatiramer acetate. 1 ml of the solution for injection (the contents of one prefilled syringe) contains 40 mg of glatiramer acetate, equivalent to 36 mg of glatiramer.
- The other ingredients are mannitol and water for injections.
Appearance and Package Contents of the Product
Copaxone prefilled syringe solution for injection is a clear, sterile solution without visible particles.
Each prefilled syringe is packaged individually in a PVC blister pack.
Copaxone is available in packs containing 3, 12, or 36 prefilled syringes of 1 ml injectable solution or a multipack of 36 prefilled syringes consisting of 3 packs, each containing 12 prefilled syringes of 1 ml injectable solution.
Only some pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
Teva GmbH
Graf Arco Strasse 3
89079 Ulm
Germany
Manufacturer
Norton Healthcare Limited T/A IVAX Pharmaceuticals UK (Teva Runcorn)
Aston Lane North, Whitehouse Vale Industrial Estate
Runcorn, Cheshire, WA7 3FA
United Kingdom
or
Actavis Group PTC ehf.
Dalshraun 1,
220 Hafnarfjörður
Iceland
or
Merckle GmBH
Graf Arco Strasse 3
Ulm - D-89079
Germany
This medicinal product is authorised in the Member States of the European Economic Area under the name COPAXONE 40 mg/ml:
Germany, Austria, Belgium, Croatia, Czech Republic, Cyprus, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, France, Greece, Hungary, Ireland, Iceland, Italy, Latvia, Lithuania, Luxembourg, Malta, Norway, Netherlands, Poland, Portugal, United Kingdom (Northern Ireland), Romania, Sweden.
Further information about this medicinal product is available on the website of the European Medicines Agency http://www.ema.europa.eu/.
You can request more information about this medicinal product by contacting the local representative of the Marketing Authorisation Holder:
Teva Pharma, S.L.U.
C/ Anabel Segura, 11, Edificio Albatros B, 1ª planta
28108, Alcobendas, Madrid (Spain)
Date of last revision of this leaflet:September 2024
Detailed and up-to-date information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
You can access detailed and up-to-date information about this medicinal product by scanning the QR code included in the packaging with your mobile phone (smartphone). You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/79515/P_79515.html
QR Code + URL
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COPAXONE 40 mg/mL injectable solution in pre-filled syringeDosage form: INJECTABLE, 20 mg glatiramer acetate / mlActive substance: glatiramer acetateManufacturer: Teva GmbhPrescription requiredDosage form: INJECTABLE, 20 mg/mlActive substance: glatiramer acetateManufacturer: Viatris LimitedPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: glatiramer acetateManufacturer: Viatris LimitedPrescription required
Online doctors for COPAXONE 40 mg/mL injectable solution in pre-filled syringe
Discuss questions about COPAXONE 40 mg/mL injectable solution in pre-filled syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















