
Remurel
Ask a doctor about a prescription for Remurel

How to use Remurel
Leaflet accompanying the packaging: patient information
Remurel, 40 mg/ml, solution for injection in a pre-filled syringe
Glatiramer acetate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Remurel and what is it used for
- 2. Important information before using Remurel
- 3. How to use Remurel
- 4. Possible side effects
- 5. How to store Remurel
- 6. Contents of the pack and other information
1. What is Remurel and what is it used for
Remurel is a medicinal product used to treat relapsing-remitting multiple sclerosis (MS). This medicine affects the way the patient's immune system works and is classified as an immunostimulant. It is believed that MS symptoms are caused by the improper functioning of the body's immune system, leading to the formation of inflammatory lesions in the brain and spinal cord.
Remurel is used to reduce the frequency of MS relapses (disease exacerbations). No beneficial effect of the medicine has been demonstrated in other forms of multiple sclerosis where relapses do not occur or occur very rarely.
Remurel may not affect the duration of an MS relapse or the severity of symptoms during a relapse.
2. Important information before using Remurel
When not to use Remurel
- if the patient is allergic to glatiramer acetate or any of the other ingredientsof this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Remurel, discuss the following with your doctor or pharmacist:
- if the patient has kidney or heart disease, regular examinations or check-ups may be necessary,
- if the patient has or has had any liver disease(including those caused by alcohol consumption). Remurel may cause severe allergic reactions, some of which can be life-threatening. These reactions can occur shortly after administration of the medicine, several months, or even several years after starting treatment, even if no allergic reactions occurred after previous administration.
Symptoms of subjective and objective allergic reactions may overlap with post-injection reactions. The doctor will inform the patient about the symptoms of an allergic reaction.
Children and adolescents
Remurel should not be used in children and adolescents under 18 years of age.
Elderly patients
No studies have been conducted on the use of Remurel in elderly patients.
Consult your doctor.
Remurel and other medicines
Tell your doctor or pharmacistabout all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to take.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor for advice and consider using Remurel during pregnancy.
Remurel can be used during pregnancy after consulting a doctor.
Limited human data do not indicate a negative effect of glatiramer acetate on breastfed infants and (or) toddlers. Remurel can be used during breastfeeding.
Driving and using machines
Remurel does not affect the ability to drive and use machines.
3. How to use Remurel
This medicine should always be used exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist.
The recommended dose for adults is one pre-filled syringe (40 mg glatiramer acetate), administered by subcutaneous injection three times a week, with at least 48 hours between injections, for example, on Mondays, Wednesdays, and Fridays. It is recommended to administer the medicine on the same days every week.
It is very important to administer Remurel correctly:
- The medicine should only be administered by subcutaneous injection (see "Instructions for use" below).
- The medicine should be used at the dose prescribed by the doctor. Only the dose prescribed by the doctor should be used.
- A pre-filled syringe should never be used more than once. Any unused product or waste should be disposed of.
- The contents of Remurel pre-filled syringes should not be mixed or administered simultaneously with any other products.
- If the solution contains visible particles, it should not be used. A new pre-filled syringe should be used.
The doctor or nurse will provide the patient with detailed instructions and will supervise the first self-administration of Remurel. The doctor or nurse will stay with the patient during and for 30 minutes after the self-administration to ensure that no adverse reactions occur.
Instructions for use
Before using Remurel, read these instructions carefully.
Before injecting the medicine, make sure you have all the necessary items:
- One blister pack containing one pre-filled syringe of Remurel.
- A container for used needles and syringes.
- For each injection, remove only one blister pack with one pre-filled syringe from the packaging. Store all other syringes in the box.
- If the syringes have been stored in the refrigerator, remove the blister pack with the syringe from the refrigerator and let it sit at room temperature for at least 20 minutes before injecting the medicine to allow the solution to reach room temperature.
Wash your hands thoroughly with soap and water.
If the patient wants to use an auto-injector to administer the injections, an auto-injector called Autoxon is available, which can be used with Remurel. The Autoxon auto-injector is designed for use with Remurel and has not been tested for use with other medicines. Read the instructions for use provided with the Autoxon auto-injector.
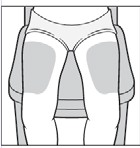
Choose an injection site from the areas indicated in the diagrams.
There are seven areas on the body where injections can be administered:
Area 1. Abdomen around the navel. Avoid injecting within a 5 cm radius of the navel,
Areas 2 and 3: Thighs (above the knees),

Areas 4, 5, 6, and 7: Back of the arms and upper hips (below the waist).
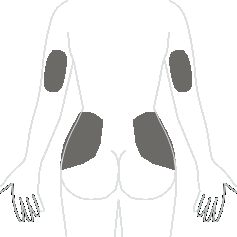
Within each area, there are several injection sites. Choose a different site for each subsequent injection to reduce the likelihood of irritation or pain at the injection site. Rotate the areas and injection sites within a given area. Do not inject the medicine in the same spot every time.
Warning:do not inject the medicine in a painful or discolored area, or in an area with lumps or nodules.
Consider developing a rotation schedule for choosing injection sites and record it in a diary. There are certain areas on the body where it is difficult to self-administer injections (e.g., the back of the arm). If the patient wants to use these areas for injections, they may need assistance from another person.
How to inject:
- Remove the syringe from the protective cap by tearing off the foil.
- Remove the needle shield without using your mouth or teeth.
- Gently grasp the skin fold between your thumb and index finger of your free hand (diagram 1).
- Insert the needle into the skin as shown in diagram 2.
- Inject the medicine by pushing the plunger down at a steady rate until the syringe is empty.
- Withdraw the syringe and needle straight up. Dispose of the syringe in a special container. Used syringes should not be thrown away in household trash. They should be carefully placed in a special container for sharp objects, following the doctor's or nurse's instructions.
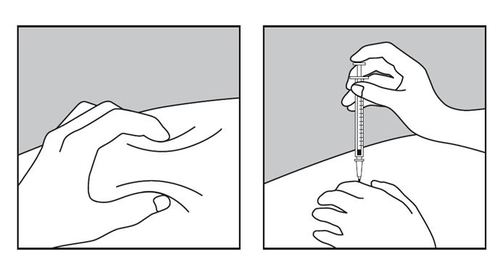
Diagram 1.
Diagram 2.
If you feel that the effect of Remurel is too strong or too weak, consult your doctor.
Using a higher dose of Remurel than recommended
Consult your doctor immediately.
Missing a dose of Remurel
Administer the dose as soon as you remember or when you are able to, then skip the dose the next day. Do not take a double dose to make up for a missed dose. If possible, return to your regular injection schedule the following week.
Stopping treatment with Remurel
Do not stop using Remurel without consulting your doctor.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions (hypersensitivity, anaphylactic reactions)
Severe allergic reactions to this medicine can occur shortly after administration. This is an uncommon side effect. These reactions can occur several months or years after starting treatment with Remurel, even if no allergic reactions occurred after previous administration.
Stop using Remurel and consult your doctor immediately or
go to the emergency department of your nearest hospitalif you notice any of the following side effects:
- widespread rash (red spots or hives);
- swelling of the eyelids, face, lips, mouth, throat, or tongue,
- sudden difficulty breathing, breathing difficulties, or wheezing,
- seizures (convulsions),
- difficulty swallowing or speaking,
- fainting, feeling dizzy or lightheaded,
- falling.
Other post-injection reactions (immediate post-injection reaction)
In some people, one or more of the following symptoms may occur within a few minutes of injecting Remurel. They usually do not cause any complications and resolve on their own within 30 minutes.
However, if the following symptoms last longer than 30 minutes, consult your doctor immediately or
go to the emergency department of your nearest hospital:
- redness of the skin (with a feeling of heat) on the chest or face (vasodilation);
- shallow breathing (dyspnea);
- chest pain;
- rapid heartbeat and fast heart rate (palpitations, tachycardia).
Liver disease
Rarely, liver disease or worsening of existing liver disease, including liver failure, can occur during treatment with Remurel. Consult your doctor immediately if you experience symptoms such as:
- nausea
- loss of appetite
- dark urine and pale stools
- yellowing of the skin or the white part of the eyes
- increased tendency to bleed.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C,
02-222 Warsaw,
phone: +48 22 49-21-301,
fax: +48 22 49-21-309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Remurel
Keep the medicine out of the sight and reach of children.
Store in a refrigerator (2°C – 8°C).
Remurel pre-filled syringes can be stored at room temperature (15°C – 25°C) for up to 1 month after removal from the refrigerator. After 1 month, if any Remurel pre-filled syringes have not been used and are still in their original packaging, they must be returned to the refrigerator.
Do not freeze.
Pre-filled syringes should be stored in the outer packaging to protect them from light.
Do not use this medicine after the expiry date stated on the label and carton after: EXP. The first two digits indicate the month, and the last four digits indicate the year.
The expiry date indicates the last day of the specified month.
If the solution contains visible particles, the syringe should be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Remurel contains
- The active substance of Remurel is glatiramer acetate. 1 ml of the solution for injection (the contents of one pre-filled syringe) contains 40 mg of glatiramer acetate.
- The other ingredients are mannitol and water for injections.
What Remurel looks like and contents of the pack
Remurel solution for injection in pre-filled syringes is a clear, colorless or slightly yellow/brownish solution.
If the solution contains visible particles, it should be discarded and a new pre-filled syringe should be used.
3 pre-filled syringes
12 pre-filled syringes
36 pre-filled syringes
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Zentiva k.s.
U kabelovny 130
Dolní Měcholupy
102 37 Prague 10
Czech Republic
Manufacturer/Importer
Synthon Hispania SL
C/ Castelló no1, Pol. Las Salinas, Sant Boi de Llobregat
08830 Barcelona
Spain
Synthon BV
Microweg 22
6545 CM Nijmegen
Netherlands
This medicine is authorized in the Member States of the European Economic Area under the following names:
Bulgaria: Ремурел 40 mg/ml инжекционен разтвор в предварително напълнена спринцовка
Czech Republic: Remurel
Croatia: Remurel 40 mg/ml otopina za injekciju, u napunjenoj štrcaljki
Estonia: Remurel
Iceland: Remurel
Latvia: Remurel 40 mg/ml šķīdums injekcijām pilnšļircē
Lithuania: Remurel 40 mg/ml injekcinis tirpalas užpildytame švirkšte
Netherlands: Glatirameeracetaat Zentiva 40 mg/ml, oplossing voor injectie in een voorgevulde spuit
Poland: Remurel
Romania: Remurel 40 mg/ml soluţie injectabilă în seringă preumplută
Slovenia: Remurel 40 mg/ml raztopina za injiciranje v napolnjeni injekcijski brizgi
Slovakia: Remurel 40 mg/ml
Hungary: Remurel 40 mg/ml oldatos injekció előretöltött fecskendőben
The auto-injector for multiple use is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Autoxon
Bulgaria, Croatia, Estonia, Iceland, Lithuania, Latvia, Poland, Czech Republic
Romania, Slovenia, Slovakia, Hungary
Sensigo
Netherlands
For more information about this medicine, contact the local representative of the marketing authorization holder:
Zentiva Polska Sp. z o.o.
Bonifraterska 17
00-203 Warsaw
phone: +48 22 375 92 00
[Zentiva (logo)]
Date of last revision of the leaflet:March 2025
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterSynthon B.V. Synthon Hispania S.L.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RemurelDosage form: Solution, 20 mg/mlActive substance: glatiramer acetatePrescription requiredDosage form: Solution, 40 mg/mlActive substance: glatiramer acetatePrescription requiredDosage form: Solution, 40 mg/mlActive substance: glatiramer acetatePrescription required
Alternatives to Remurel in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Remurel in Ukraine
Alternative to Remurel in Spain
Online doctors for Remurel
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Remurel – subject to medical assessment and local rules.














