
Glatiramer acetate Teva
Ask a doctor about a prescription for Glatiramer acetate Teva

How to use Glatiramer acetate Teva
Leaflet accompanying the packaging: patient information
Glatiramer acetate Teva, 40 mg/ml, solution for injection in a pre-filled syringe
Glatiramer acetate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Glatiramer acetate Teva and what is it used for
- 2. Important information before using Glatiramer acetate Teva
- 3. How to use Glatiramer acetate Teva
- 4. Possible side effects
- 5. How to store Glatiramer acetate Teva
- 6. Contents of the packaging and other information
1. What is Glatiramer acetate Teva and what is it used for
Glatiramer acetate Teva is a medicine used to treat relapsing forms of multiple sclerosis, administered three times a week. Glatiramer acetate Teva is not indicated for use in patients with primary or secondary progressive multiple sclerosis. It modifies the patient's immune system and is classified as an immunomodulatory drug. It is believed that the symptoms of multiple sclerosis are caused by an immune system disorder, leading to the formation of inflammatory lesions in the brain and spinal cord. Glatiramer acetate Teva is used to reduce the frequency of multiple sclerosis relapses (exacerbations). No effect of the medicine has been demonstrated in other forms of multiple sclerosis where relapses are rare or do not occur. Glatiramer acetate Teva may not affect the duration of a multiple sclerosis relapse or the severity of symptoms during the relapse.
2. Important information before using Glatiramer acetate Teva
When not to use Glatiramer acetate Teva:
- if the patient is allergic to glatiramer acetate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Glatiramer acetate Teva, the patient should discuss it with their doctor or pharmacist:
- if the patient has kidney or heart disease, as the doctor may order regular laboratory tests and check-ups.
- if the patient has or has had any liver problems (including those caused by alcohol consumption).
Children and adolescents
Glatiramer acetate Teva should not be used in children and adolescents under 18 years of age.
Elderly patients
The use of Glatiramer acetate Teva has not been studied in elderly patients. The patient should consult their doctor.
Glatiramer acetate Teva and other medicines
The patient should tell their doctor or pharmacist about all medicines they are taking, have recently taken, or plan to take.
Pregnancy and breastfeeding
If the patient is pregnant, thinks they may be pregnant, or plans to have a baby, they should consult their doctor before using this medicine. Limited data in humans have not shown a negative effect of Glatiramer acetate Teva on breastfed infants. Glatiramer acetate Teva can be used during breastfeeding.
Driving and using machines
The effect of Glatiramer acetate Teva on the ability to drive and use machines is not known.
3. How to use Glatiramer acetate Teva
This medicine should always be used exactly as prescribed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist. The usual dose for adults is one pre-filled syringe (40 mg of glatiramer acetate), administered subcutaneously three times a week, at intervals of at least 48 hours. It is recommended to administer the medicine on the same days every week. When the patient receives Glatiramer acetate Teva for the first time, the doctor or nurse will provide full instructions and supervise the patient's condition. The doctor or nurse will stay with the patient while they administer the injection themselves and for another half hour to ensure that no adverse reactions occur.
Instructions for use
Before using Glatiramer acetate Teva, the patient should read these instructions carefully. Before injecting the medicine, the patient should make sure they have all the necessary items:
- One blister of Glatiramer acetate Teva, pre-filled syringe. For each injection, remove only one blister with one pre-filled syringe from the packaging. Store all other syringes in the packaging.
- If the syringes were stored in the refrigerator, remove the blister with the syringe from the refrigerator and let it stand at room temperature for at least 20 minutes before injecting the medicine, allowing the solution to warm up to room temperature.
- A container for used needles and syringes.
The patient should wash their hands thoroughly with soap and water. If the patient wants to use an injector, they can use the CSYNC device for Glatiramer acetate Teva injections. This injector is an approved device for use with Glatiramer acetate Teva and has not been tested with other products. The patient should read the instructions for use provided with the CSYNC injector. The patient should choose an injection site using the diagrams. There are seven areas on the body where injections can be administered:
- Area 1:The abdominal area around the navel. Avoid injecting within a 5 cm radius of the navel.
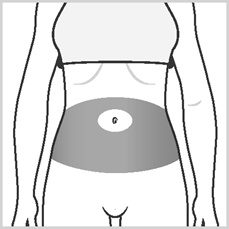
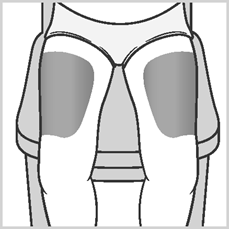
- Areas 2 and 3:Thighs (above the knees),
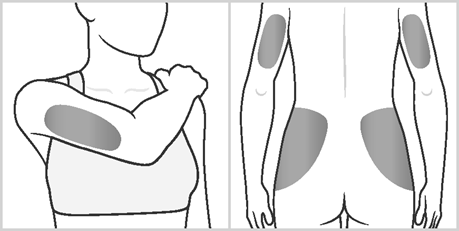
- Areas 4, 5, 6, and 7:Upper arms and hips (below the waist).
In each injection area, there are multiple injection sites. The patient should choose a different site for each injection to reduce the risk of irritation or pain at the injection site. The patient should rotate the injection sites within the same area. The patient should not inject the medicine in the same spot every time.Warning:the patient should not inject the medicine in a painful area or where the skin is discolored, has lumps, or has a rash. It is recommended to develop a scheme for rotating injection sites and record it in a diary. There are certain areas on the body where it is difficult to administer injections by oneself (e.g., the back of the arm). In such cases, assistance is needed. How to inject the medicine:
- Remove the syringe from the protective blister by tearing off the foil.
- Remove the needle cap, do notremove the cap with the mouth or teeth.
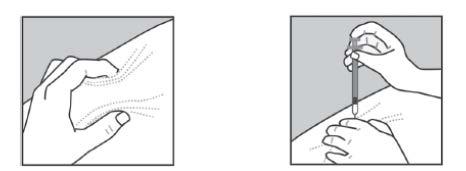
- Gently grasp the skin fold between the thumb and index finger of the free hand (Figure 1).
- Insert the needle into the skin as shown in Figure 2.
- Inject the medicine by pressing the plunger down slowly until the syringe is empty.
- Withdraw the syringe and needle in an upward motion.
- Dispose of the syringe in a special container.
Figure 1 Figure 2
It is very important to administer Glatiramer acetate Teva correctly:
- Administer only by subcutaneous injection.
- Use the dose prescribed by the doctor. The patient should only use the dose prescribed by the doctor.
- Never use the same pre-filled syringe more than once. Unused product or waste should be disposed of.
- Glatiramer acetate Teva pre-filled syringe should not be mixed or administered with other medicines.
- If the solution contains visible particles, it should not be used. A new syringe should be used.
If the patient feels that the effect of Glatiramer acetate Teva is too strong or too weak, they should consult their doctor.
Using a higher dose of Glatiramer acetate Teva than recommended
The patient should inform their doctor immediately.
Missing a dose of Glatiramer acetate Teva
The patient should administer the dose as soon as they remember or as soon as they are able to, and skip the next day's dose. The patient should not take a double dose to make up for the missed dose. If possible, the patient should return to their regular injection schedule the following week.
Stopping treatment with Glatiramer acetate Teva
The patient should not stop using Glatiramer acetate Teva without consulting their doctor. If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions (hypersensitivity)
Rarely (may affect up to 1 in 1000 patients), a severe allergic reaction to the medicine may occur.
The patient should stop using Glatiramer acetate Teva and contact their doctor immediately or go to the emergency department of the nearest hospital
if they experience any of the following symptoms:
- rash (red spots or hives)
- swelling of the eyelids, face, or lips
- sudden shortness of breath
- seizures (convulsions)
- fainting.
Other reactions after injection (immediate post-injection reaction)
In some patients, within a few minutes of injecting Glatiramer acetate Teva, reactions with at least one of the following symptoms may occur, although not very often (in up to 1 in 100 patients). These reactions usually do not cause any problems and resolve within half an hour. However, if the following symptoms persist for more than 30 minutes, the patient should contact their doctor immediately or go to the emergency department of the nearest hospital:
- flushing (redness of the skin with a feeling of heat) on the chest or face (vasodilation)
- shortness of breath (dyspnea)
- chest pain
- rapid or irregular heartbeat (palpitations, tachycardia).
Liver disease
Rarely, liver disease or worsening of existing liver disease, including liver failure in some cases requiring liver transplantation, may occur during treatment with Glatiramer acetate Teva. The patient should contact their doctor immediately if they experience symptoms such as:
- nausea
- loss of appetite
- dark urine and pale stools
- yellowing of the skin or the white part of the eyes
- increased tendency to bleed.
Side effects reported by patients using glatiramer acetate 40 mg/ml administered three times a week
Side effects reported by patients using glatiramer acetate 20 mg/ml (see below) were also reported in patients using glatiramer acetate 40 mg/ml.
Very common: may affect more than 1 in 10 patients
- infection, flu
- anxiety, depression
- headache
- nausea
- skin rash
- joint or back pain
- weakness, injection site reactions, including redness, pain, swelling, itching, and induration (these reactions at the injection site are common and usually decrease over time), non-specific pain.
Common: may affect up to 1 in 10 patients
- respiratory tract infection, gastroenteritis, herpes, ear infection, rhinitis, dental abscess, thrush
- benign skin growth (benign skin tumor), growth (tumor)
- lymph node enlargement
- allergic reactions
- loss of appetite, weight gain
- irritability
- taste disorders, increased muscle tone, migraine, speech disorders, fainting, tremor
- double vision, eye disorders
- ear disorders
- cough, hay fever
- anal or rectal disorders, constipation, tooth decay, dyspepsia, swallowing difficulties, fecal incontinence, vomiting
- abnormal liver function test results
- bruises, excessive sweating, itching, skin disorder, hives
- neck pain
- urinary urgency, frequent urination, inability to empty the bladder properly
- chills, facial swelling, atrophy of subcutaneous tissue at the injection site, local reaction, peripheral edema due to fluid accumulation, fever
Uncommon: may affect up to 1 in 100 patients
- abscess, skin and subcutaneous tissue infections, furuncles, shingles, kidney infection
- skin cancer
- increased white blood cell count, decreased white blood cell count, splenomegaly, decreased platelet count, changes in the appearance of white blood cells
- goiter, hyperthyroidism
- decreased alcohol tolerance, gout, increased blood lipid levels, increased blood sodium levels, decreased serum ferritin levels
- strange dreams, confusion, euphoric mood; seeing, hearing, smelling, or tasting something that does not exist (hallucinations), hostility, excessively good mood, personality disorders, suicidal behavior
- carpal tunnel syndrome (numbness and pain in the hands), mental disorders, seizures (convulsions), agraphia, muscle disorders, movement disorders, muscle spasms, neuritis, neuromuscular junction disorders leading to muscle dysfunction, rapid involuntary eye movements, paralysis, foot drop (peroneal nerve palsy), loss of consciousness (stupor), blurred vision
- cataract, corneal damage, dry eye sensation, eye bleeding, ptosis, mydriasis, optic nerve atrophy leading to vision disorders
- extrasystoles, bradycardia, occasional tachycardia
- varicose veins
- periodic breathing pauses, nosebleeds, abnormally rapid or deep breathing (hyperventilation), feeling of throat constriction, respiratory disorders, inability to breathe due to throat constriction (feeling of choking)
- enteritis, colon polyp, belching, esophageal ulcer, gingivitis, rectal bleeding, salivary gland enlargement
- cholelithiasis, liver enlargement
- skin and subcutaneous tissue swelling, contact dermatitis, painful red nodules on the skin, skin nodules
- edema, arthritis, or arthralgia (joint or hip pain), joint or tendon sheath inflammation (tenosynovitis), hip pain, muscle atrophy
- hematuria, kidney stones, urinary disorders, abnormal urine
- breast swelling, erectile dysfunction, pelvic organ prolapse, prolonged erection, prostate disorders, abnormal Pap smear (abnormal cervical smear), testicular disorders, vaginal bleeding, vaginal disorders
- cyst, signs of alcohol abuse, hypothermia, unspecified inflammation, tissue necrosis at the injection site, mucosal disorders
- vaccination-related disorders.
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, PL-02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Glatiramer acetate Teva
The medicine should be stored out of the sight and reach of children. The medicine should not be used after the expiry date stated on the outer packaging and on the immediate packaging after "Expiry date:" or "EXP:". The expiry date refers to the last day of the month stated. The medicine should be stored in a refrigerator (2°C - 8°C). Glatiramer acetate Teva pre-filled syringe can be stored at room temperature (15°C - 25°C) for a single period of up to 1 month. After 1 month, if the pre-filled syringes have not been used and are still in their original packaging, they must be returned to the refrigerator. The medicine should not be frozen. The pre-filled syringes should be stored in their original packaging to protect them from light. The patient should dispose of all syringes containing solid particles. Used syringes should be disposed of carefully, using a puncture-resistant container, in accordance with the doctor's or nurse's instructions. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Glatiramer acetate Teva contains
- The active substance of the medicine is glatiramer acetate. 1 ml of the solution for injection (the contents of one pre-filled syringe) contains 40 mg of glatiramer acetate.
- The other ingredients are mannitol and water for injections.
What Glatiramer acetate Teva looks like and contents of the packaging
Glatiramer acetate Teva is a clear solution for injection in a pre-filled syringe. Glatiramer acetate Teva is available in packs of 3 or 12 pre-filled syringes, each containing 1 ml of the solution for injection, or in a multipack containing 3 cartons, each with 12 pre-filled syringes containing 1 ml of the solution for injection. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer/importer
Marketing authorization holder: Teva GmbH, Graf-Arco-Str. 3, 89079 Ulm, Germany. Manufacturer/Importer: Teva Pharmaceuticals Europe B.V., Swensweg 5, 2031 GA Haarlem, Netherlands; Merckle GmbH, Ludwig-Merckle-Strasse 3, 89143 Blaubeuren, Germany; Actavis Group PTC ehf., Dalshraun 1, 220, Hafnarfjörður, Iceland.
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany: Glatirameracetat AbZ 40 mg/ml Injektionslösung in einer Fertigspritze, Austria: Copaxobene 40 mg/ml Injektionslösung in einer Fertigspritze, Belgium: Glataxon 40 mg/ml solution injectable en seringue préremplie / oplossing voor injectie in een voor gevulde spuit / Injektionslösung in einer Fertigspritze, Luxembourg: Glaxaton 40 mg/ml solution injectable en seringue préremplie, Poland: Glatiramer acetate Teva, Portugal: Acetato de Glatirâmero Mepha, Slovakia: Glatirameracetát Teva 40 mg/ml injekčný roztok naplnený v injekčnej striekačke. For more information, the patient should contact the representative of the marketing authorization holder: Teva Pharmaceuticals Polska Sp. z o.o., ul. Emilii Plater 53, 00-113 Warsaw, tel.: (22) 345 93 00.
Date of last revision of the leaflet: July 2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterActavis Group PTC ehf. Actavis Group PTC ehf. Merckle GmbH Teva Pharmaceutical Europe B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Glatiramer acetate TevaDosage form: Solution, 20 mg/mlActive substance: glatiramer acetatePrescription requiredDosage form: Solution, 40 mg/mlActive substance: glatiramer acetatePrescription requiredDosage form: Solution, 20 mg/mlActive substance: glatiramer acetatePrescription required
Alternatives to Glatiramer acetate Teva in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Glatiramer acetate Teva in Ukraine
Alternative to Glatiramer acetate Teva in Spain
Online doctors for Glatiramer acetate Teva
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Glatiramer acetate Teva – subject to medical assessment and local rules.














