
COLIROFTA ATROPINE 10 mg/ml EYE DROPS SOLUTION

How to use COLIROFTA ATROPINE 10 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What COLIROFTA ATROPINA 10 mg/ml is and what it is used for
- What you need to know before starting to use COLIROFTA ATROPINA 10 mg/ml
- How to use COLIROFTA ATROPINA 10 mg/ml
- Possible adverse effects
- Conservation of COLIROFTA ATROPINA 10 mg/ml
- Contents of the package and additional information
Introduction
Prospect:information for the user
COLIROFTA ATROPINA 10 mg/ml eye drops in solution
Read the entire prospectus carefully before starting to use this medication, as it contains important information for you.
|
Contents of the prospectus
- What COLIROFTA ATROPINA 10 mg/ml is and what it is used for
- What you need to know before starting to use COLIROFTA ATROPINA 10 mg/ml
- How to use COLIROFTA ATROPINA 10 mg/ml
- Possible adverse effects
5 Conservation of COLIROFTA ATROPINA 10 mg/ml
- Contents of the package and additional information
1. What COLIROFTA ATROPINA 10 mg/ml is and what it is used for
It is an eye drop that contains atropine, an anticholinergic agent (blocks some of the acetylcholine receptors, a neurotransmitter) that, when administered in the eyes, produces mydriasis (pupil dilation) and cycloplegia (paralysis of the muscle that produces accommodation).
Colirofta Atropina 10 mg/ml is indicated:
- In eye exams, to dilate the pupil and prevent eye focusing.
- For the treatment of acute inflammatory eye conditions in the anterior part of the eye, such as inflammation of the colored part of the eye (iritis) and of the iris and ciliary body (iridocyclitis).
2. What you need to know before starting to use COLIROFTA ATROPINA 10 mg/ml
Do not use COLIROFTA ATROPINA 10 mg/ml
- If you are allergic to atropine or any of the other components of this medication (listed in section 6).
- If you have or think you may have primary glaucoma or a predisposition to narrow-angle glaucoma (increased pressure in the eye).
- Children with Down syndrome, spastic paralysis (a type of cerebral palsy), or brain injury.
- Children under 3 years of age.
- Children who have had a previous severe reaction to the active ingredient of this medication, atropine.
(See section 2, "Pregnancy, breastfeeding, and fertility").
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colirofta Atropina 10 mg/ml.
- Use this medication only in your eye(s).
- After application, keep your eyes closed while gently pressing the tear duct with your finger for at least 1 minute. See section 3 "How to use...".
- The use of this medication may cause:
- Increased pressure in the eye and glaucoma, especially in elderly patients. Eye pressure should be monitored frequently and also before starting treatment, under medical supervision.
- Changes in behavior (such as delirium), especially in children and elderly patients, although these reactions can occur at any age.
- Sensitivity to light. Protect your eyes from intense light (sunglasses).
- Blurred vision that can last up to 2 weeks.
- If you have a fever or are exposed to high temperatures, especially in children, caution is required, as this medication can increase body temperature.
- Great caution is needed in patients with heart disease, arrhythmias, or recent myocardial infarction, as tachycardias (increased heart rate) may occur. It is recommended to reduce the dose.
It may cause an increase in blood pressure.
- Cautious use is required in patients with:
- significant kidney disease
- chronic pulmonary diseases
- or symptoms of the lower urinary tract, such as prostate hyperplasia.
- Do not use this medication at the same time as treatment with MAOI medications (usually antidepressants) (see "Other medications...").
Children
- This medication will be used with caution in children over 3 years of age.
- Premature children and those with low birth weight or patients with Down syndrome, spastic paralysis, brain injury, or children with fair skin and blue eyes are especially sensitive to the adverse effects of this medication. Consult your doctor to inform you about the serious adverse reactions that may occur with the use of this medication.
- Avoid children putting this medication in their mouth or cheeks. Wash your hands and the children's cheeks or hands immediately after administration.
Other medications and COLIROFTA ATROPINA 10 mg/ml
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Especially, tell your doctor if you are using:
Medications that share properties with the active ingredient of the medication, such as:
- Amantadine (antiviral medication and for Parkinson's disease, which stimulates the central nervous system)
- For obstructive pulmonary diseases, such as: tiotropium, glycopyrronium, or revefenacin
- Scopolamine (used previously to anesthesia)
- Certain antihistamines (medications used to treat allergies)
- Antipsychotics (used to treat psychiatric conditions)
- Tricyclic antidepressants (medications used to treat depression).
Other medications:
- Donepezil (used in Alzheimer's disease)
- Heart stimulants
- MAOI medications (generally used to treat depression).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
This medication is not recommended during pregnancy or breastfeeding.
Driving and using machines
The influence of this medication on the ability to drive and use machines is significant.
This medication can cause blurred vision and sensitivity to light for a prolonged period, which can last several days. Do not drive or use machines or tools until your vision is clear.
COLIROFTA ATROPINA 10 mg/ml containsmethylparahydroxybenzoate (E-218) and propylparahydroxybenzoate (E-216) and phosphates
This medication may cause allergic reactions (possibly delayed) because it contains methylparahydroxybenzoate (E-218) and propylparahydroxybenzoate (E-216).
This medication contains 6 mg of phosphates per ml.
If you have severe corneal damage (the transparent layer of the front of the eye), treatment with phosphates, in very rare cases, can cause blurred vision due to calcium accumulation.
3. How to use COLIROFTA ATROPINA 10 mg/ml
Follow the administration instructions of this medication indicated by your doctor or pharmacist exactly. If in doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults
When a sustained effect is intended, three daily applications of 2 drops are usually performed.
For refraction examination, 1 or 2 drops will be instilled in each eye twice a day, 1 to 3 days before the examination.
Use in children
Children (over 3 years of age)
Due to the risk of producing severe systemic adverse effects, this medication is contraindicated in children under 3 years of age and is recommended to be used with caution in children over 3 years. The lowest effective dose possible is recommended to reduce the risk of systemic adverse effects (see "Warnings and precautions").
When a sustained effect is intended, three daily applications of 1 drop are usually performed.
For refraction examination, 1 or 2 drops will be instilled in each eye twice a day, 1 to 3 days before the examination.
Remember to apply your medication according to your doctor's instructions.
Use in elderly patients
Use with caution in elderly patients, as they may have a higher risk of undiagnosed glaucoma and behavioral disorders.
Your doctor will indicate the duration of your treatment with Colirofta Atropina 10 mg/ml. Do not stop treatment before, unless your doctor indicates it.
Recommendations for use:
Ophthalmic route (in the eyes).
1 2 3
- Wash your hands.
- Take the bottle.
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
- Hold the bottle, upside down, between your fingers.
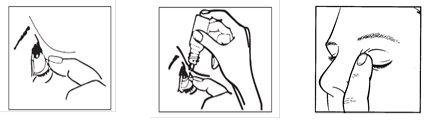
- Tilt your head back. Gently separate the eyelid from the eye with your finger until a pocket forms between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, or nearby areas, with the dropper. The drops may become contaminated.
- Gently squeeze the base of the bottle with your index finger to release one drop at a time (figure 2).
- After using this eye drop, release the lower eyelid, close your eye, and gently press the edge of the eye, next to the nose, for at least 1 minute. This helps prevent the medication from entering the rest of the body (figure 3).
- If you are applying drops in both eyes, repeat all the previous steps with the other eye.
- Close the bottle tightly immediately after use.
- Remember to wash your hands after administering this medication.
If a drop falls outside the eye, try again.
If you are using other ophthalmic medications, wait at least 5 minutes between the administration of this eye drop and other ophthalmic medications. Ophthalmic ointments should be administered last.
If you use more COLIROFTA ATROPINA 10 mg/ml than you should
You can eliminate it by washing your eyes with warm water. Do not apply more drops until it is time for your next dose. The symptoms of overdose may include redness and dryness of the skin (in children, it may present as a rash), blurred vision, rapid and irregular pulse, fever, abdominal swelling in children, convulsions, hallucinations, loss of coordination, and rapid progressive respiratory depression.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service. Phone: 91 562 04 20, indicating the medication and the amount used.
If you forget to use COLIROFTA ATROPINA 10 mg/ml
Do not apply a double dose to make up for forgotten doses.
Apply a single dose as soon as you remember and continue with the next dose that was scheduled. However, if it is almost time for the next dose, do not apply the forgotten dose and continue with the next dose of your regular regimen.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medications, this medication can cause adverse effects, although not all people experience them.
The following reactions have been reported during post-marketing experience:
Frequency not known(cannot be estimated from the available data):
- Ocular effects:blurred vision, increased pupil size (prolonged effect of the medication), eyelid swelling, sensitivity to light.
- General effects: allergy, increased or decreased heart rate, headache, dizziness, hallucination, confusion, disorientation, intestinal obstruction, abdominal swelling, vomiting, redness or inflammation of the skin, rash, fever.
Other adverse effects in children
Children are more likely to exhibit the general adverse effects described above, especially in premature children and those with low birth weight or patients with Down syndrome, spastic paralysis, or brain injury (see section 2, "Do not use...").
Reporting adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaRAM.es.By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of COLIROFTA ATROPINA 10 mg/ml
Keep this medication out of the sight and reach of children.
Store below 25°C.
Do not use this medication after the expiration date that appears on the bottle and on the box after EXP. The expiration date is the last day of the month indicated.
To avoid infections, the bottle must be discarded 4 weeks after it was first opened.
Write the opening date of the bottle in the box provided for this purpose.
Medications should not be thrown away in drains or trash. Deposit the packaging and medications you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Contents of the package and additional information
Composition of COLIROFTA ATROPINA 10 mg/ml
- The active ingredient is atropine. One ml of eye drop solution contains 10 mg of atropine (as sulfate) (1%).
- The other components are: methylparahydroxybenzoate (E-218), propylparahydroxybenzoate (E-216), sodium chloride, sodium hydrogen phosphate dodecahydrate, potassium dihydrogen phosphate, and purified water.
Appearance of the product and contents of the package
Colirofta Atropina 10 mg/ml is an eye drop solution; it is a clear and colorless liquid that comes in a 10 ml dropper bottle (plastic bottle) with a sealed cap.
Marketing authorization holder and manufacturer
Marketing authorization holder
Alcon Healthcare S.A.
World Trade Center Almeda Park
Plaça de la Pau s/n, Edificio 6, planta 3
08940 - Cornellà de Llobregat (Barcelona)
Spain
Manufacturer
Siegfried El Masnou, S.A.
C/ Camil Fabra, 58
08320 El Masnou – Barcelona
Spain
or
Alcon Laboratories Belgium
Lichterveld 3
2870 Puurs-Sint-Amands
Belgium
Date of the last revision of this prospectus:May 2020
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price5.39 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLIROFTA ATROPINE 10 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 5 mgActive substance: atropineManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: EYEDROP, 10 mgActive substance: cyclopentolateManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: EYEDROP, 10 mg/mlActive substance: tropicamideManufacturer: Alcon Healthcare S.A.Prescription required
Online doctors for COLIROFTA ATROPINE 10 mg/ml EYE DROPS SOLUTION
Discuss questions about COLIROFTA ATROPINE 10 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions










